Keeling SO, Alabdurubalnabi Z, Avina-Zubieta A, et al. Canadian Rheumatology Association Recommendations for the Assessment and Monitoring of Systemic Lupus Erythematosus. J Rheumatol. 2018 Oct;45(10):1426-1439. doi: 10.3899/jrheum.171459. Epub 2018 Sep 1. PubMed PMID: 30173152.
van Vollenhoven RF, Mosca M, Bertsias G, et al. Treat-to-target in systemic lupus erythematosus: recommendations from an international task force. Ann Rheum Dis. 2014 Jun;73(6):958-67. doi: 10.1136/annrheumdis-2013-205139. Epub 2014 Apr 16. PubMed PMID: 24739325.
Ruiz-Irastorza G, Cuadrado MJ, Ruiz-Arruza I, et al. Evidence-based recommendations for the prevention and long-term management of thrombosis in antiphospholipid antibody-positive patients: report of a task force at the 13th International Congress on antiphospholipid antibodies. Lupus. 2011 Feb;20(2):206-18. doi: 10.1177/0961203310395803. PubMed PMID: 21303837.
Bertsias GK, Ioannidis JP, Aringer M, et al. EULAR recommendations for the management of systemic lupus erythematosus with neuropsychiatric manifestations: report of a task force of the EULAR standing committee for clinical affairs. Ann Rheum Dis. 2010 Dec;69(12):2074-82. doi: 10.1136/ard.2010.130476. Epub 2010 Aug 19. PubMed PMID: 20724309.
Mosca M, Tani C, Aringer M, et al. European League Against Rheumatism recommendations for monitoring patients with systemic lupus erythematosus in clinical practice and in observational studies. Ann Rheum Dis. 2010 Jul;69(7):1269-74. doi: 10.1136/ard.2009.117200. Epub 2009 Nov 5. PubMed PMID: 19892750; PubMed Central PMCID: PMC2952401.
Bertsias G, Ioannidis JP, Boletis J, et al; Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics. EULAR recommendations for the management of systemic lupus erythematosus. Report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics. Ann Rheum Dis. 2008 Feb;67(2):195-205. Epub 2007 May 15. PubMed PMID: 17504841.
Definition, Etiology, PathogenesisTop
Systemic lupus erythematosus (SLE) is a complex autoimmune disease leading to chronic inflammation of various organs and tissues. The etiology is unknown.
Clinical Features and Natural HistoryTop
Women are affected 6 to 10 times more frequently than men. In almost two-thirds of patients disease onset is between the ages of 16 and 55 years, although it can also present initially in children or the elderly. The presenting symptoms may be nonspecific, such as fever, malaise, or weight loss. SLE usually presents as a multisystem disease, though disease manifestations can sometimes be limited to a single organ, such as the skin or kidneys. The course is typically relapsing-remitting in nature; however, a complete resolution of clinical and laboratory manifestations can occur.
1. Systemic manifestations: Fatigue, low-grade or high-grade fever, weight loss.
2. Cutaneous manifestations:
1) Acute cutaneous lupus erythematosus (ACLE) develops in 60% to 80% of patients with SLE; it may be limited to the face, involving the cheeks and nose (“butterfly rash”: Figure 1), or the lesions may be located on the forehead, around the eyes, or on the neck or chest. The lesions are frequently triggered by ultraviolet light, and the photosensitivity usually manifests within 24 hours of sun exposure. Rashes can persist from days to weeks. Acute cutaneous lupus can take several forms, including generalized erythroderma (Figure 2), papular or vesicular lesions, or it may be severe and bullous in nature, resembling toxic epidermal necrolysis. In patients with active disease, ulcerations of the nasal and oral mucosa are frequently found. These ulcerations can be painful or painless.
2) Subacute cutaneous lupus erythematosus (SCLE) affects ~20% of patients with SLE and is a particular form of nonscarring photosensitive rash typically associated with anti-Sjögren syndrome A (anti-SSA) antibodies (also called anti-Ro antibodies). The lesions usually appear in one of 2 forms: circular, often raised, with a clear central area (Figure 3); or scaly, psoriatic-like patches. As the lesions are typically associated with sun exposure, they are usually located on the neck, arms, and chest. While they do not cause scarring, they may be responsible for abnormal pigmentation or telangiectasia.
3) Chronic cutaneous lupus erythematosus (CCLE) is a noteworthy subgroup of cutaneous lupus, as it is often present in isolation, without any other systemic symptoms of lupus. Such patients are described as having isolated CCLE without having SLE. The most common variant of CCLE is discoid lupus erythematosus (DLE), which is characterized by erythematous or violaceous scaly plaques typically occurring on the head and neck, leading to follicular plugging, scarring, and localized alopecia (Figure 4). Early epidemiologic studies indicated that only 5% to 10% of patients with DLE would progress to fulfill the criteria for SLE, however, a more recent study has quoted a figure of >15%; incidence rates of SLE vary, depending on definition. Other CCLE rashes are hypertrophic (verrucous) lupus, lupus panniculitis (also called lupus profundus), chilblains lupus, and lupus erythematosus tumidus.
4) Other, nonspecific cutaneous manifestations include alopecia, lichen myxedematosus, atrophia maculosa cutis, and neutrophilic dermatoses.
5) Vascular cutaneous manifestations can be subdivided into those caused by true vasculitis (pathologic findings consistent with leukocytoclastic vasculitis or panarteritis) and those classified as a nonspecific vasculopathy. Cutaneous leukocytoclastic vasculitis can present clinically as palpable purpura with or without ulcerative lesions or as urticarial vasculitis. Panarteritis can present similarly or with subcutaneous nodules resembling polyarteritis nodosa. Vasculopathy-type manifestations include Raynaud phenomenon (in 15%-40% of patients), livedo reticularis, cutaneous ulceration/necrosis, palmar erythema (particularly of the thenar and hypothenar eminences), fingertip erythema, nail-fold telangiectasias, erythromelalgia, splinter hemorrhages, Osler nodes, and Janeway lesions. The presence of cutaneous vascular lesions has been associated with a higher overall SLE disease activity. Skin ulcers, livedo reticularis, splinter hemorrhages, and fingertip erythema have been found to be associated with antiphospholipid antibodies.
3. Musculoskeletal manifestations: More than 80% of patients complain of arthralgia and/or myalgia, which are often migratory and of variable severity. A smaller number of patients develops a true inflammatory arthritis, typically affecting the small joints of the hands and/or feet; when the hands are affected, it usually involves the metacarpophalangeal (MCP), proximal interphalangeal (PIP), and wrist joints, and can be deforming. Although in these characteristics it can be indistinguishable from rheumatoid arthritis (RA), unlike RA it is nonerosive; this deforming, nonerosive arthritis is termed Jaccoud arthropathy. Tendonitis and tendon rupture as well as joint laxity are reported more frequently in patients with SLE than in the general population.
Patients with SLE can also develop generalized myositis, with elevated creatine kinase (CK) levels and inflammatory or necrotic changes on muscle biopsy, often similar to the changes found in dermatomyositis or polymyositis. Myositis is important to differentiate from myalgia, as only proven myositis warrants the introduction or augmentation of high-dose glucocorticoid and/or immunomodulatory therapy.
4. Renal manifestations (lupus nephritis): see Lupus Nephritis.
5. Pulmonary manifestations: There are several different lung manifestations of SLE. Pleuritis with or without pleural effusion is a common manifestation occurring in up to 50% of patients. Pulmonary fibrosis of the nonspecific interstitial fibrosis pattern is a well-recognized SLE complication. Pulmonary hypertension can either occur in isolation or be associated with another pulmonary manifestation, such as venous thromboembolic disease. Diffuse alveolar hemorrhage can result secondary to interstitial lung disease with diffuse alveolar damage or autoimmune capillaritis. Shrinking lung syndrome, characterized by dyspnea, elevated hemidiaphragm(s), and restrictive findings on pulmonary function testing in the absence of parenchymal disease, is a rare but well-recognized complication. Pulmonary embolism occurs, particularly in patients with antiphospholipid antibodies. Acute interstitial pneumonitis can occur rarely (though mortality is high), and chronic fibrotic lung disease can result. Pulmonary complications of dysregulated immunity and immunomodulatory therapy should be considered in the differential diagnosis of patients presenting with respiratory symptoms and signs, including respiratory infections, as well as adverse effects of therapy, such as pneumonitis or interstitial lung disease secondary to methotrexate use.
6. Cardiovascular manifestations include pericarditis with pericardial effusion, myocarditis (rare, usually asymptomatic, found on echocardiography as global contractility impairment in patients with unexplained tachycardia or nonspecific ST-segment and T-wave abnormalities; it may cause conduction disturbances), valvular lesions possibly leading to valve dysfunction (often associated with antiphospholipid antibodies), noninfective (Libman-Sacks) endocarditis, hypertension (due to renal involvement or as a complication of glucocorticoid treatment), and increased risk of early development of coronary artery disease.
7. Nervous system manifestations (neuropsychiatric systemic lupus erythematosus [NPSLE]) are reported in 6% to 60% of patients, depending on the cohort and the NPSLE definition used. The American College of Rheumatology (ACR) has described 19 neuropsychiatric syndromes, subdivided into central and peripheral nervous system disorders as follows:
1) Central nervous system: Aseptic meningitis, cerebrovascular disease (ischemic stroke, transient ischemic attack), demyelinating syndrome, headache, movement disorder (eg, chorea), myelopathy, seizure disorder, acute confusional state, anxiety disorder, cognitive dysfunction, mood disorder, psychosis.
2) Peripheral nervous system: Acute inflammatory demyelinating polyneuropathy (Guillain-Barré syndrome), autonomic disorder, mononeuropathy (simplex or multiplex), myasthenia gravis, cranial neuropathy, plexopathy, polyneuropathy.
To date, no laboratory or imaging findings specific to NPSLE have been described, and attribution of neurologic or psychiatric symptoms to SLE can be difficult. The most common abnormality on magnetic resonance imaging (MRI) in patients who present with NPSLE is nonspecific high T2-signal intensity lesions in white matter that can be focal or diffuse. These lesions have been found to be associated with antiphospholipid antibodies. Cerebrospinal fluid can show elevated protein levels with elevated IgG index. When patients present with neurologic, psychiatric, or neuropsychological findings, physicians have to consider and exclude alternative causes, such as infections, metabolic disturbances, and adverse effects of therapy.
8. Hematologic manifestations: Common hematologic abnormalities include leukopenia, lymphopenia, anemia, and thrombocytopenia (see Diagnostic Tests, below). Clinically, lymphadenopathy is common; lymph nodes are usually soft, painless, and not fixed to the surrounding tissues. Splenomegaly can be found. Secondary thrombotic thrombocytopenic purpura can rarely occur.
9. Gastrointestinal manifestations are relatively uncommon in SLE and can include dysphagia, hepatomegaly, or aseptic peritonitis. Mesenteric or other intra-abdominal thromboses can occur in association with antiphospholipid antibodies.
DiagnosisTop
Diagnosis is based on the typical clinical features and results of diagnostic tests. Negative antinuclear antibody (ANA) tests by immunofluorescence make the diagnosis of SLE much less likely (they are positive in >95% of patients), while positive anti–double-stranded DNA (dsDNA) or anti-Sm antibodies usually confirm the diagnosis. In 2019, new SLE classification criteria were published by the European League Against Rheumatism (EULAR) and ACR (Table 1). Note that classification criteria are designed mostly to classify patients for research purposes and may clinically misclassify some patients diagnosed in routine clinical practice.
1. Laboratory tests:
1) Serum biochemistry:
a) Inflammatory markers: Neither the erythrocyte sedimentation rate (ESR) nor C-reactive protein (CRP) is a reliable indicator of disease activity in SLE. In patients with polyclonal gammopathy, the ESR can be chronically elevated even in the absence of disease activity.
b) Hemoglobin: An inflammatory anemia (anemia of chronic disease) is commonly found in patients with SLE, characterized by elevated ferritin levels with low serum iron, iron saturation, and total iron-binding capacity. Hemolytic anemia with a positive Coombs test result is characteristic of SLE and is an indication for systemic glucocorticoid and/or immunomodulatory therapy.
c) White blood cells: Leukopenia (15%-20% of patients) and lymphopenia <1.5×109/L are common in SLE and usually do not require specific therapy. Severe neutropenia may require therapy.
d) Platelets: Thrombocytopenia is common and can be caused either by immunologic disturbances associated with SLE or by secondary antiphospholipid syndrome.
e) Creatinine: Creatinine levels can be elevated in class III, IV, or V lupus nephritis.
f) Hypergammaglobulinemia: Hypergammaglobulinemia is generally polyclonal. It may or may not be associated with active disease.
2) Urinalysis: Proteinuria is generally present in class III, IV, and V lupus nephritis; hematuria or pyuria can also be present, particularly in class III and IV nephritis. Urine sediment examination in class III and IV lupus nephritis can reveal dysmorphic erythrocyte, leukocyte, and erythrocyte casts.
3) Immunology: More than 95% of patients with SLE have ANAs, including extractable nuclear antigens (ENAs) by immunofluorescence or newer immunoassays (BioPlex). ANAs/ENAs represent a group of autoreactive antibodies directed against the nucleus. ENAs constitute antibodies to specific nuclear components, and these reactivities may predict clinical subtypes/phenotypes of lupus. The anti-dsDNA and anti-Sm antibodies are highly specific (95%-97%) for SLE diagnosis. Drug-induced SLE is associated with antihistone antibodies in >95% of people. Some autoantibodies are more specific to the involvement of certain organs, for instance: anti-dsDNA, lupus nephritis; anti-RNP, myositis; anti-SSA/anti-Ro, lymphopenia, lymphadenopathy, SCLE, sicca complex. Higher disease activity can correlate with low levels of C3 or C4 complement components and elevated levels of anti-dsDNA; in particular, all these can accompany activity of lupus nephritis. Antiphospholipid antibodies (anticardiolipin, anti–beta2-glycoprotein I, nonspecific inhibitor) are found in ~30% of patients with SLE.
2. Biopsies:
1) Skin: Skin biopsy samples taken from the areas with evident erythematous lesions or even from apparently healthy skin can reveal immunoglobulin and complement deposits along the border of the epidermis and dermis, although these may also occur in other skin conditions as well as in 20% of healthy individuals.
2) Renal: Kidney biopsy is indicated in the majority of patients with features of lupus nephritis. Biopsy results identify the type of glomerular lesions as well as the activity and chronic character of renal involvement, which has implications for both management and prognosis (see Lupus Nephritis).
Other connective tissue diseases that can coexist or may be confused with SLE include Sjögren syndrome, systemic sclerosis, dermatomyositis/polymyositis, drug-induced SLE, mixed connective tissue disease (MCTD), and undifferentiated connective tissue disease (UCTD). However, depending on the specific clinical manifestations under consideration, differential diagnosis may be very broad and can include a variety of different conditions. For example, hematological abnormalities may be due to drug-induced lupus (causes: Table 2), myeloproliferative disorders (particularly lymphomas), idiopathic thrombocytopenic purpura, microangiopathic hemolytic anemia, antiphospholipid syndrome, or infections. Facial rash may need to be differentiated from rosacea, seborrheic dermatitis, photodermatoses, and dermatomyositis. Conditions that may be associated with positive autoantibodies: Table 2.
TreatmentTop
The therapy of SLE varies based on specific clinical manifestations. While treatments of some specific manifestations of SLE (eg, lupus nephritis) have been reasonably well studied, management of many manifestations of SLE is hindered by a lack of high-quality comparative evidence. Thus, medication use in SLE is guided in many cases by uncontrolled studies, case series, and expert opinion. Guidance from a specialist physician with expertise in SLE management may be necessary.
1. Nonpharmacologic treatment: It is important for patients with SLE to avoid exposure to ultraviolet radiation by avoiding sunlight as well as tanning beds. Ultraviolet light is associated with both cutaneous SLE activity and increased overall SLE disease activity.
2. Pharmacologic treatment: Glucocorticoids are very effective for acute management of active SLE and are indicated in all patients with moderate or severe disease manifestations. However, given the potential adverse effects of glucocorticoids, concomitant use of other immunomodulatory agents is indicated in virtually all patients with SLE. The choice of medications and their dosage depends on predominant clinical features and disease activity.
1) Antimalarials: Hydroxychloroquine 5 mg/kg (usual maximum dose, 400 mg daily) or, if hydroxychloroquine is not tolerated, chloroquine 250 to 500 mg daily is indicated in all patients with SLE.Evidence 1Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). Moderate Quality of Evidence (moderate confidence that we know true effects of intervention). Quality of Evidence lowered due to imprecision. For further information, see Appendix 1 at the end of the chapter. All patients taking antimalarial agents require screening for the rare risk of associated antimalarial-induced retinopathy. The timing of screening is variable, based on risk of retinopathy, and ranges from every 6 months to annually. Low-risk patients can generally be effectively screened by an optometrist. However, higher-risk patients and patients in whom a concern regarding hydroxychloroquine-induced retinopathy has been raised should be evaluated by an ophthalmologist.
2) Methotrexate: Methotrexate is often used to achieve disease control in patients in whom glucocorticoids cannot be tapered off, or for whom disease activity is incompletely controlled with glucocorticoids and hydroxychloroquine. Typical manifestations thought to be controlled by methotrexate include arthritis, cutaneous manifestations, and serositis. Typical doses range from 15 to 25 mg weekly administered orally or subcutaneously. Patients need to be monitored for liver enzyme elevation and cytopenias on a monthly basis or more frequently in higher-risk situations. Other adverse effects include potential development of interstitial lung disease or the risk of acute hypersensitivity pneumonitis. Methotrexate is teratogenic and should not be used in pregnant patients.
3) Azathioprine: Azathioprine is often used to achieve disease control in patients who are unable to taper off glucocorticoids or in whom disease activity is incompletely controlled with glucocorticoids and hydroxychloroquine. Typical manifestations thought to be controlled by azathioprine include cutaneous manifestations, serositis, and hematological abnormalities. It is also used as maintenance therapy for renal disease. Typical doses range from 1 to 3 mg/kg/d. Patients need to be monitored for cytopenias and liver enzyme elevation, initially every 2 weeks for 2 months, and monthly thereafter if no adverse events have been identified. Cytopenias can pose a particular risk in patients who have low or deficient thiopurine methyltransferase (TPMT) activity, and TMPT genotyping can be considered prior to the initiation of therapy with azathioprine.
4) Mycophenolate mofetil (MMF)/mycophenolic acid: MMF is the ester prodrug of mycophenolic acid. These agents are used to control disease in SLE, particularly in patients with active class III, IV, or severe class V lupus nephritis (see Lupus Nephritis). It may have some benefit in the management of cutaneous manifestations and hematologic abnormalities in SLE, though there have been few studies addressing these particular manifestations. Doses differ depending on the agent used. When used for induction therapy for lupus nephritis, MMF is typically administered at 2 to 3 g/d in divided doses. Patients should be monitored for cytopenias on a monthly basis, or more frequently in higher-risk situations. Other adverse effects include elevation in cholesterol levels and gastrointestinal intolerance. The teratogenic potential of MMF is unknown, and at this time it is not recommended for use in pregnancy.
5) Cyclophosphamide: Cyclophosphamide was first introduced in the treatment of class III or IV lupus nephritis (see Lupus Nephritis) in combination with glucocorticoids, either in pulse or daily dose regimens. Because of its potentially irreversible adverse effects on fertility, currently its use in SLE as a potent immunosuppressive agent is typically reserved for rapidly progressive nephritis unresponsive to MMF and for other severe, potentially life-threatening manifestations of SLE, including severe cutaneous vasculitis, diffuse alveolar hemorrhage, interstitial pneumonitis, NPSLE, or hematological manifestations, such as refractory autoimmune hemolytic anemia. Cyclophosphamide has a risk of causing cytopenias, particularly leukopenia. If it is being administered intravenously, a complete blood count should be performed at the time of white blood cell nadir at 7 to 10 days after administration and every 2 weeks thereafter. A repeat administration should not be given until the white blood cell count has recovered. For oral cyclophosphamide, the complete blood count should be monitored at least every 2 weeks or more frequently.
6) Rituximab: There has been controversy regarding the role of rituximab in SLE. At this time, use of rituximab is generally limited to special circumstances only.
7) Belimumab: Belimumab is a monoclonal antibody against anti–B lymphocyte-stimulating factor administered as an IV infusion. It has recently received an approval for use as an addition to the standard therapy for active nonrenal, nonneuropsychiatric SLE manifestations. Belimumab is used particularly to attempt to spare glucocorticoids in patients who have been unable to taper off glucocorticoids to an acceptable level.
Treatment of Specific Organ Manifestations
1. Cutaneous manifestations:
1) Avoidance of sunlight exposure: Protective clothing, sunscreens with a sun protection factor ≥15.
2) Topical treatment: Glucocorticoid ointments and creams (fluorinated glucocorticoids cause skin atrophy and should be used for short periods) or calcineurin inhibitors (eg, 0.1% tacrolimus).
3) Systemic treatment: Antimalarial agents, methotrexate, retinoids (eg, isotretinoin), other agents (eg, thalidomide, dapsone, mycophenolate mofetil, azathioprine), intravenous immunoglobulin (IVIG), and biological agents (eg, rituximab) may also be used.
2. Hematologic manifestations:
1) Autoimmune hemolytic anemia and autoimmune thrombocytopenia usually respond well to glucocorticoids. Other agents that may be effective include azathioprine, MMF, cyclosporine (INN ciclosporin), cyclophosphamide, IVIG, and rituximab. In treatment-resistant patients, splenectomy may be considered.
2) Leukopenia usually does not require treatment. In the case of granulocyte counts <0.5×109/L, consider granulocyte colony-stimulating factor (G-CSF).
3) Thrombotic thrombocytopenic purpura.
4) Macrophage activation syndrome: see Special Considerations, below.
3. Arthralgia, myalgia, and arthritis: For arthralgia, myalgia, and arthritis, nonsteroidal anti-inflammatory drugs (NSAIDs) and antimalarial agents are the mainstays of therapy. For severe inflammatory arthritis in the acute phase, glucocorticoids are typically effective, and patients generally respond to doses of <15 mg of prednisone or equivalent daily. Methotrexate is often effective in patients with arthritis with incomplete response to antimalarial agents or dependence on prednisone.
4. Serositis: In patients with acute manifestations NSAIDs can be used for mild serositis, while glucocorticoids at a low to moderate dose (up to 40 mg/d, then the dose is tapered off) may be necessary for refractory symptoms or large associated effusions. Antimalarial agents, methotrexate, and azathioprine are also effective.
5. Renal manifestations: see Lupus Nephritis.
6. NPSLE:
1) Neurologic symptoms in a patient with SLE should prompt a search for other underlying causes other than SLE itself; it is important to consider and exclude infection, drug reaction, and metabolic disturbances prior to instituting or amplifying immunosuppressive therapy.
2) The mainstay of therapy for active severe NPSLE symptoms, such as acute psychosis, seizures, myelopathy, peripheral neuropathy, or acute inflammatory demyelinating polyneuropathy, are high-dose glucocorticoids (a pulse of 500-1000 mg IV methylprednisolone once daily for 3 consecutive days, then 1 mg/kg to a maximum of 60 mg/d and subsequently tapered off) in combination with cyclophosphamide.
3) If the neurologic symptoms are thrombotic in origin, such as thrombotic stroke, and if they are associated with antiphospholipid antibodies (APLAs), antiplatelet and/or anticoagulant drugs may be the most appropriate management (see Antiphospholipid Syndrome).
4) Adjunctive treatment can be used as appropriate to treat the individual NPSLE manifestations. For example, antiepileptic drugs are indicated in the management of seizures, and antipsychotic medications may be indicated in acute psychosis.
Treatment of Drug-Induced Lupus
1. Discontinue the drug that has caused the symptoms; in most cases, this will lead to the resolution of symptoms within a few days. The exception to this is hydralazine, in which case a more prolonged course of symptoms can occur.
2. In rare cases, depending on the severity of clinical manifestations, the use of NSAIDs and/or glucocorticoids and/or antimalarial agents for a limited time may be necessary.
3. Additional interventions:
1) Osteoporosis prophylaxis in patients treated with glucocorticoids.
2) Addressing cardiovascular risk factors, given the associated risk of early cardiovascular disease.
3) Vaccinations, particularly influenza and pneumococcal vaccines. Other vaccinations may be considered on the basis of an individual risk. Live vaccines are often contraindicated in patients with SLE due to the use of immunomodulatory agents.
4) Women of reproductive age require counseling about contraception and family planning. The following tenets should be considered:
a) Estrogen-containing oral contraceptives do not increase disease activity in SLE in patients with well-controlled disease.Evidence 4High Quality of Evidence (high confidence that we know true effects of intervention). For more information, see Appendix 4 at the end of the chapter.
b) Estrogen-containing contraceptives should be avoided in patients with APLA due to the increased thrombosis risk.Evidence 5Strong recommendation (downsides clearly outweigh benefits; right action for all or almost all patients). Low Quality of Evidence (low confidence that we know true effects of the intervention). Quality of Evidence lowered due to the lack of experimental data. For more information, see Appendix 5 at the end of the chapter.
c) Many immunomodulatory agents used in SLE are teratogenic and must be stopped for an appropriate interval prior to any attempt at conception (depending on the medication, this interval can be <6 months).
Attempt at conception should ideally be made only after there has been clinical and serological quiescence of SLE activity for >6 months.Evidence 6Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). Low Quality of Evidence (low confidence that we know true effects of the intervention). Quality of Evidence lowered due to the observational nature of data. For more information, see Appendix 6 at the end of the chapter.
5) In patients with persistently high titers of APLA, consider antiplatelet agents and/or hydroxychloroquine (see Antiphospholipid Syndrome).
Follow-UpTop
1. In patients with complete remission and no evidence of target organ damage or comorbidities, follow-up visits every 6 to 12 months are recommended. The remaining patients should be followed up more frequently.
2. Estimate the activity of SLE and diagnose recurrences on the basis of clinical symptoms, laboratory parameters (complete blood count, creatinine and albumin levels, proteinuria, urine sediment, C3 and C4 complement levels, and anti-dsDNA titers), and general SLE activity indices (eg, systemic lupus erythematosus disease activity index [SLEDAI]). These should be followed up every 3 to 12 months, depending on the clinical situation.
3. APLA levels should be measured before an intended pregnancy, surgery, or estrogen therapy.
4. Patients should undergo a diagnostic workup of hepatitis B virus, hepatitis C virus, cytomegalovirus, and tuberculosis infections based on the individual risk, particularly before starting an intensive immunosuppressive treatment.
Special ConsiderationsTop
Macrophage Activation Syndrome
Macrophage activation syndrome (MAS) is one of the acquired forms of hemophagocytic lymphohistiocytosis (HLH) that occurs in patients with rheumatic disorders, most commonly with systemic idiopathic juvenile arthritis and in adults with SLE. It manifests with increased and prolonged activity of macrophages and T cells (particularly CD8+), leading to an uncontrolled inflammatory response. The manifestations include fever, hepatomegaly, splenomegaly, lymphadenopathy, cytopenias, elevated liver enzymes, disseminated intravascular coagulation, hypofibrinogenemia, hyperferritinemia, and hypertriglyceridemia.
Diagnosis is based on the general HLH criteria; however, these criteria have not been validated in patients with SLE. MAS requires differentiation from sepsis, malignancy, and exacerbations of SLE.
Treatment is controversial, and there is little evidence to guide therapy in this condition. Management typically consists of initially high-dose glucocorticoids, along with varying combinations of IVIG, cyclosporine, cyclophosphamide, tacrolimus, or etoposide. Poor prognostic factors include infection and CRP levels >50 mg/L.
SLE does not affect fertility but it is associated with risks related to pregnancy and the health of both the mother and the child. It is recommended that female patients do not become pregnant until remission has been achieved. Flares occur in ~30% of pregnant patients with SLE; they are more likely to occur and can be more severe in patients in whom good control of disease activity was not achieved prior to conception. Antimalarial agents, glucocorticoids, and azathioprine are generally considered safe in pregnancy and are typically continued. NSAIDs have been associated with an increased risk of spontaneous abortion in the first trimester as well as with premature closure of the ductus arteriosus in the third trimester; however, they are generally considered safe in the second trimester. Obstetric complications and preeclampsia are mainly associated with APLA and lupus nephritis. The presence of anti-Ro and anti-La antibodies in the mother may cause neonatal lupus (in 3% of affected pregnancies) and is associated with cardiac conduction defects and congenital heart block. Patients with anti-Ro and/or anti-La antibodies should be followed by a high-risk obstetrical team and the fetus should be screened with fetal echocardiogram at intervals during pregnancy. Breastfeeding is possible, though care must be taken to use only medications compatible with breastfeeding.
Before surgery disease activity should be assessed, as surgery may worsen the course of SLE. It is recommended to achieve remission before surgery, unless it cannot be postponed. The most rapid improvement can be achieved with perioperative administration of glucocorticoids.
PrognosisTop
The most common causes of death in patients with early SLE include infections and severe target organ involvement (central nervous system, cardiovascular system, acute lupus pneumonitis, severe nephropathy); in patients with long-standing disease, these include complications of treatment (infections) and the effects of accelerated atherosclerosis as well as thromboembolism. With appropriate diagnosis and treatment, the 10-year overall survival rate is 80% to 98% depending on age at diagnosis, specific cohort studied, and country of origin, and the 20-year survival rate is ~65%. More than 40% to 50% of patients develop some degree of permanent target organ damage after 5 years of follow-up. In lupus nephritis, 10% to 30% of patients develop end-stage renal failure over 15 years despite treatment. Recurrence of SLE in transplanted kidneys is very rare (2%).
Tables and FiguresTop
|
Only one criterion, with the highest score, should count per each domain. It is sufficient to meet each criterion once and not necessarily simultaneously. Do not count a given criterion if it is likely explained by other conditions. Classification as SLE requires ≥10 points and ≥1 clinical criterion present. | |||
|
Entry criterion: ANA titer ≥1/80a | |||
|
Clinical domain |
Criteria |
Points |
Comments |
|
Fever |
Fever >38.3°C |
2 |
|
|
Mucous membrane and skin |
Acute cutaneous lupus |
6 |
Observed by a clinician (direct examination or review of photographs) |
|
Subacute cutaneous or discoid lupus |
4 | ||
|
Oral ulcers |
2 | ||
|
Nonscarring alopecia |
2 | ||
|
Musculoskeletal |
Joint involvement |
6 |
≥2 joints involved with synovitis (swelling/effusion) or Tenderness and ≥30 min of morning stiffness |
|
Serous membranes |
Acute pericarditis |
6 |
|
|
Pleural or pericardial effusion |
5 |
On any of ultrasonography, radiography, CT, or MRI | |
|
Renal involvement |
Renal biopsy class III or IV |
10 |
|
|
Renal biopsy class II or V |
8 |
| |
|
Proteinuria >0.5 g/24 h |
4 |
| |
|
Neurologic and psychiatric |
Seizures |
5 |
Generalized or partial/focal |
|
Psychosis |
3 |
Delusions or hallucinations without insight and with absence of delirium | |
|
Delirium |
2 |
| |
|
Hematologic |
Autoimmune hemolysis |
4 |
Positive Coombs (direct antiglobulin) test and Low haptoglobin, elevated indirect bilirubin, elevated LDH |
|
Thrombocytopenia <100×109/L |
4 |
| |
|
Leukopenia <4×109/L |
3 |
| |
|
SLE-specific antibodies |
Anti-dsDNA or anti-Sm |
6 |
|
|
Antiphospholipid antibodies |
Anti-cardiolipin, anti-beta2GPI, lupus anticoagulant |
2 |
|
|
Complement protein |
Low C3 and low C4 |
4 |
|
|
Low C3 or low C4 |
3 |
| |
|
a Testing using HEp-2 cells or equivalent. | |||
|
ACR, American College of Rheumatology; ANA, antinuclear antibody; CT, computed tomography; dsDNA, double-stranded DNA; EULAR, European League Against Rheumatism; GPI, glycoprotein I; LDH, lactate dehydrogenase; MRI, magnetic resonance imaging; SLE, systemic lupus erythematosus. | |||
| Conditions | Examples |
|
Systemic connective tissue disorders |
Systemic lupus erythematosus, scleroderma, Sjögren syndrome, polymyositis/dermatomyositis, mixed connective tissue disease, antiphospholipid syndrome, rheumatoid arthritis |
|
Adverse drug reactions (including drug-induced lupus) |
Chlorpromazine, methyldopa, hydralazine, propylthiouracil, procainamide, isoniazid, minocycline, D-penicillamine, quinidine, sulfonamides, nitrofurantoin, acebutolol |
|
Chronic liver diseases |
Autoimmune hepatitis, primary biliary cholangitis, primary sclerosing cholangitis, alcoholic liver disease |
|
Chronic pulmonary diseases |
Idiopathic pulmonary fibrosis, asbestosis, primary pulmonary hypertension |
|
Infections |
Tuberculosis, syphilis, chronic HCV/HBV infection, parasitic infestations |
|
Malignancy |
Lymphoma, leukemia, melanoma, ovarian cancer, breast cancer, lung cancer, colorectal cancer, prostatic cancer |
|
Hematologic disorders |
Immune thrombocytopenic purpura, autoimmune hemolytic anemia |
|
Healthy individuals |
More common in female patients, in pregnancy, and in advanced age |
|
Other |
Diabetes, Graves disease, Hashimoto thyroiditis, multiple sclerosis, subacute endocarditis, following organ transplant |
|
HBV, hepatitis B virus; HCV, hepatitis C virus. | |
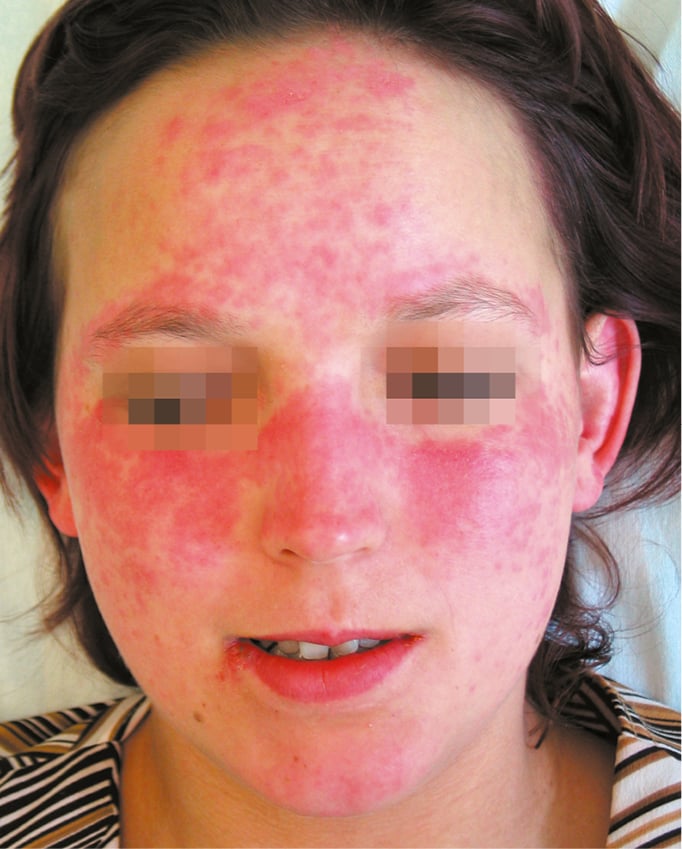
Figure 18.24-1. Systemic lupus erythematosus. Typical “butterfly” rash.
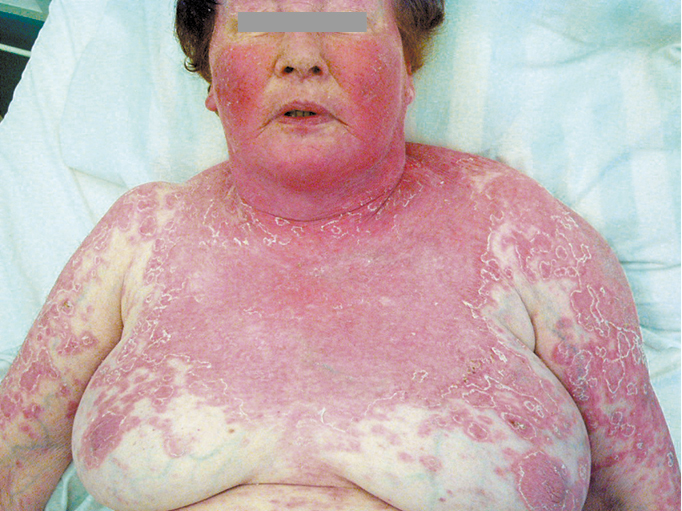
Figure 18.24-2. Systemic lupus erythematosus. Severe cutaneous manifestations following sunlight exposure.
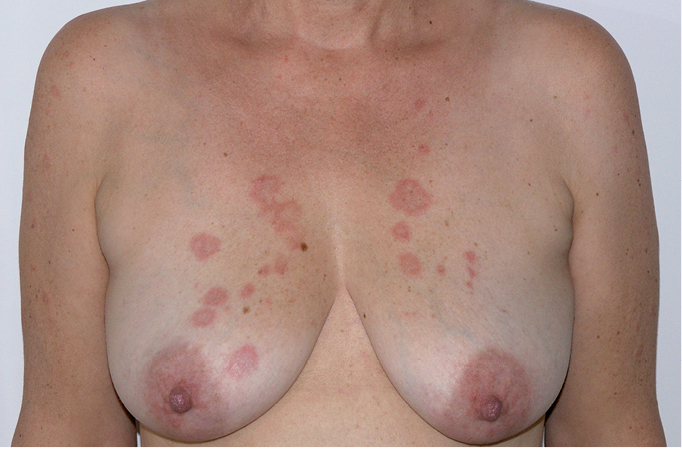
Figure 18.24-3. Subacute cutaneous lupus erythematosus. Circular lesions with erythema and swelling.
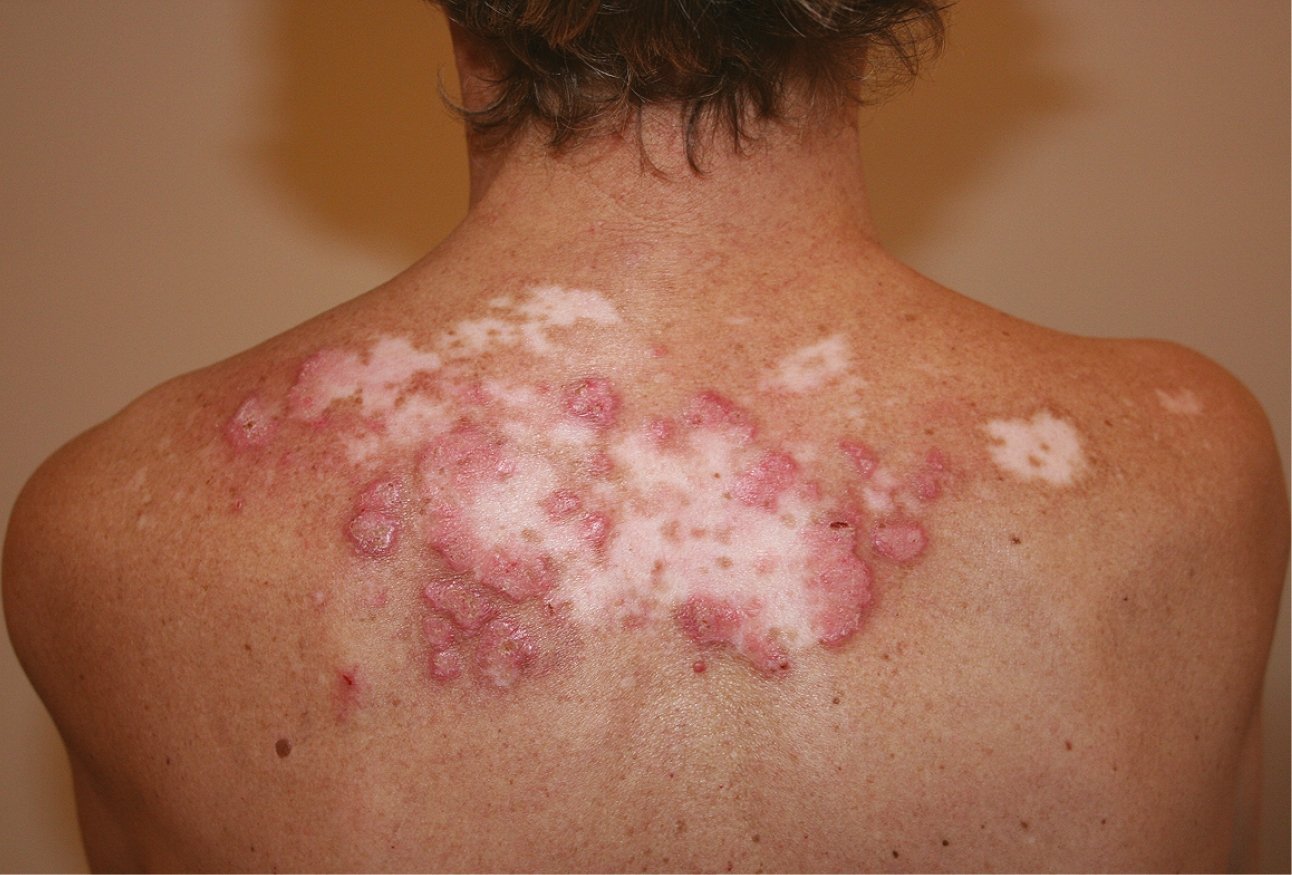
Figure 18.24-4. Discoid lupus erythematosus. Well-demarcated lesions with erythema, swelling, scarring, and central atrophy.
AppendiXTop
Multiple observational and small randomized controlled trials (RCTs) have shown the benefit of antimalarials in multiple different domains of SLE management. They have been found to be associated with lower overall disease activity, lower disease activity in specific organ systems, lower damage accrual rates, improved pregnancy outcomes, and improvement in serologic parameters, as follows. Studies have been summarized in a review by Ruiz-Irastorza et al. in 2010, and the authors recommended the use of hydroxychloroquine in all patients with SLE.1 There is some evidence in observational studies and small RCTs to suggest improved disease activity and a lower risk of flares of SLE with hydroxychloroquine.2,3 Lower hydroxychloroquine blood levels have been associated with higher disease activity,4,5 though notably treating with hydroxychloroquine to a target concentration of >1000 ng/mL did not alter the flare risk.6 SLE-associated skin disease has been shown to be improved with the use of antimalarials.7 There is some evidence for prevention of thrombosis in patients with SLE with and without APLAs,8,9 and the use of hydroxychloroquine was associated with a lower risk of vascular events in a retrospective study.10 Several retrospective observational studies have shown that hydroxychloroquine use was associated with less damage accrual in patients with SLE,11,12 and others have shown a lower risk of skin and renal damage in SLE.13,14 Two retrospective analyses have indicated that hydroxychloroquine may prevent congenital heart block in pregnancies where the fetus is exposed to anti-SSA/anti-SSB antibodies.15 Many serologic parameters are improved in patients with SLE on antimalarials. Interferon-alpha levels are lower in patients on hydroxychloroquine, and this has correlated with improvement in disease activity.3 A small RCT has shown improvement in lipid levels,16 and this has been echoed in an observational study.17 There was also a lower risk of persistently positive antiphospholipid antibodies in patients on hydroxychloroquine in 1 study.18 One RCT in a small cohort of pregnant patients revealed improved disease activity and lower prednisone doses in patients taking hydroxychloroquine,19 and several reviews of hydroxychloroquine in pregnant patients with autoimmune diseases concluded that it appears to be safe to continue during pregnancy.20,21,22
The evidence is graded as moderate quality. The studies are either observational in nature or small RCTs. The evidence was downgraded from high-quality evidence due to the small size of the RCTs, resulting in imprecision on the estimates of disease activity and flare risk.
This statement was given a strong recommendation because when taken together, the evidence provides a compelling picture supporting the use of hydroxychloroquine for all patients with SLE, particularly as the toxicity associated with its use is minimal.
1 Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, Khamashta MA. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis. 2010 Jan;69(1):20-8. doi: 10.1136/ard.2008.101766. Epub. Review. PubMed PMID: 19103632.
2 A randomized study of the effect of withdrawing hydroxychloroquine sulfate in systemic lupus erythematosus. The Canadian Hydroxychloroquine Study Group. N Engl J Med. 1991 Jan 17;324(3):150-4. PubMed PMID: 1984192.
3 Willis R, Seif AM, McGwin G Jr, et al. Effect of hydroxychloroquine treatment on pro-inflammatory cytokines and disease activity in SLE patients: data from LUMINA (LXXV), a multiethnic US cohort. Lupus. 2012 Jul;21(8):830-5. doi: 10.1177/0961203312437270. Epub 2012 Feb 17. PubMed PMID: 22343096; PubMed Central PMCID: PMC3808832.
4 Francès C, Cosnes A, Duhaut P, et al. Low blood concentration of hydroxychloroquine in patients with refractory cutaneous lupus erythematosus: a French multicenter prospective study. Arch Dermatol. 2012 Apr;148(4):479-84. doi: 10.1001/archdermatol.2011.2558. PubMed PMID: 22508872.
5 Costedoat-Chalumeau N, Amoura Z, Hulot JS, et al. Low blood concentration of hydroxychloroquine is a marker for and predictor of disease exacerbations in patients with systemic lupus erythematosus. Arthritis Rheum. 2006 Oct;54(10):3284-90. PubMed PMID: 17009263.
6 Costedoat-Chalumeau N, Galicier L, Aumaître O, et al; Group PLUS. Hydroxychloroquine in systemic lupus erythematosus: results of a French multicentre controlled trial (PLUS Study). Ann Rheum Dis. 2013 Nov;72(11):1786-92. doi: 10.1136/annrheumdis-2012-202322. Epub 2012 Nov 10. PubMed PMID: 23144449.
7 Yokogawa N, Tanikawa A, Amagai M, et al. Response to hydroxychloroquine in Japanese patients with lupus-related skin disease using the cutaneous lupus erythematosus disease area and severity index (CLASI). Mod Rheumatol. 2013 Mar;23(2):318-22. doi: 10.1007/s10165-012-0656-3. Epub 2012 May 12. Erratum in: Mod Rheumatol. 2014 Jul;24(4):701. PubMed PMID: 22581095.
8 Jung H, Bobba R, Su J, et al. The protective effect of antimalarial drugs on thrombovascular events in systemic lupus erythematosus. Arthritis Rheum. 2010 Mar;62(3):863-8. doi: 10.1002/art.27289. PubMed PMID: 20131232.
9 Tektonidou MG, Laskari K, Panagiotakos DB, Moutsopoulos HM. Risk factors for thrombosis and primary thrombosis prevention in patients with systemic lupus erythematosus with or without antiphospholipid antibodies. Arthritis Rheum. 2009 Jan 15;61(1):29-36. doi: 10.1002/art.24232. PubMed PMID: 19116963.
10 Becker-Merok A, Nossent J. Prevalence, predictors and outcome of vascular damage in systemic lupus erythematosus. Lupus. 2009 May;18(6):508-15. doi: 10.1177/0961203308099233. PubMed PMID: 19395452.
11 Akhavan PS, Su J, Lou W, Gladman DD, Urowitz MB, Fortin PR. The early protective effect of hydroxychloroquine on the risk of cumulative damage in patients with systemic lupus erythematosus. J Rheumatol. 2013 Jun;40(6):831-41. doi: 10.3899/jrheum.120572. Epub 2013 Apr 15. PubMed PMID: 23588942.
12 Fessler BJ, Alarcón GS, McGwin G Jr, et al; LUMINA Study Group. Systemic lupus erythematosus in three ethnic groups: XVI. Association of hydroxychloroquine use with reduced risk of damage accrual. Arthritis Rheum. 2005 May;52(5):1473-80. PubMed PMID: 15880829.
13 Pons-Estel GJ, Alarcón GS, González LA, et al; Lumina Study Group. Possible protective effect of hydroxychloroquine on delaying the occurrence of integument damage in lupus: LXXI, data from a multiethnic cohort. Arthritis Care Res (Hoboken). 2010 Mar;62(3):393-400. doi: 10.1002/acr.20097. PubMed PMID: 20391486; PubMed Central PMCID: PMC3202433.
14 Pons-Estel GJ, Alarcón GS, McGwin G Jr, et al; Lumina Study Group. Protective effect of hydroxychloroquine on renal damage in patients with lupus nephritis: LXV, data from a multiethnic US cohort. Arthritis Rheum. 2009 Jun 15;61(6):830-9. doi: 10.1002/art.24538. PubMed PMID: 19479701; PubMed Central PMCID: PMC2898742.
15 Izmirly PM, Costedoat-Chalumeau N, Pisoni CN, et al. Maternal use of hydroxychloroquine is associated with a reduced risk of recurrent anti-SSA/Ro-antibody-associated cardiac manifestations of neonatal lupus. Circulation. 2012 Jul 3;126(1):76-82. doi: 10.1161/CIRCULATIONAHA.111.089268. Epub 2012 May 24. PubMed PMID: 22626746; PubMed Central PMCID: PMC3437628.
16 Kavanaugh A, Adams-Huet B, Jain R, Denke M, McFarlin J. Hydroxychloroquine Effects on Lipoprotein Profiles (the HELP trial): A Double-Blind, Randomized, Placebo-Controlled, Pilot Study In Patients With Systemic Lupus Erythematosus. J Clin Rheumatol. 1997 Feb;3(1):3-8. PubMed PMID: 19078110.
17 Cairoli E, Rebella M, Danese N, Garra V, Borba EF. Hydroxychloroquine reduces low-density lipoprotein cholesterol levels in systemic lupus erythematosus: a longitudinal evaluation of the lipid-lowering effect. Lupus. 2012 Oct;21(11):1178-82. doi: 10.1177/0961203312450084. Epub 2012 May 28. PubMed PMID: 22641182.
18 Broder A, Putterman C. Hydroxychloroquine use is associated with lower odds of persistently positive antiphospholipid antibodies and/or lupus anticoagulant in systemic lupus erythematosus. J Rheumatol. 2013 Jan;40(1):30-3. doi: 10.3899/jrheum.120157. Epub 2012 Aug 1. PubMed PMID: 22859353; PubMed Central PMCID: PMC3768146.
19 Levy RA, Vilela VS, Cataldo MJ, et al. Hydroxychloroquine (HCQ) in lupus pregnancy: double-blind and placebo-controlled study. Lupus. 2001;10(6):401-4. PubMed PMID: 11434574.
20 Sperber K, Hom C, Chao CP, Shapiro D, Ash J. Systematic review of hydroxychloroquine use in pregnant patients with autoimmune diseases. Pediatr Rheumatol Online J. 2009 May 13;7:9. doi: 10.1186/1546-0096-7-9. PubMed PMID: 19439078; PubMed Central PMCID: PMC2690583.
21 Costedoat-Chalumeau N, Amoura Z, Duhaut P, et al. Safety of hydroxychloroquine in pregnant patients with connective tissue diseases: a study of one hundred thirty-three cases compared with a control group. Arthritis Rheum. 2003 Nov;48(11):3207-11. PubMed PMID: 14613284.
22 Costedoat-Chalumeau N, Amoura Z, Huong DL, Lechat P, Piette JC. Safety of hydroxychloroquine in pregnant patients with connective tissue diseases. Review of the literature. Autoimmun Rev. 2005 Feb;4(2):111-5. Epub 2004 Dec 14. Review. PubMed PMID: 15722258.
Three systematic reviews have been performed comparing MMF with IV cyclophosphamide in induction therapy for lupus nephritis. The authors have reached the same conclusion, stating that MMF is equally effective in achieving complete renal remission, partial renal remission, stable renal function, and complete remission of proteinuria.1,2,3 Rates of death and serious infection were not different between groups, though rates of ovarian failure, alopecia, and leukopenia were higher with cyclophosphamide. Both the ACR as well as the EULAR/ERA-EDTA recommend the use of either MMF or IV cyclophosphamide in addition to glucocorticoids for induction therapy. For patients in whom preservation of ovarian function is important, MMF should be considered over cyclophosphamide.
This evidence was graded as high-quality as it is based on well-designed RCTs with a low risk of bias. The evidence has further been summarized in systematic reviews.
1 Henderson LK, Masson P, Craig JC, et al. Induction and maintenance treatment of proliferative lupus nephritis: a meta-analysis of randomized controlled trials. Am J Kidney Dis. 2013 Jan;61(1):74-87. doi: 10.1053/j.ajkd.2012.08.041. Epub 2012 Nov 22. PubMed PMID: 23182601.
2 Touma Z, Gladman DD, Urowitz MB, Beyene J, Uleryk EM, Shah PS. Mycophenolate mofetil for induction treatment of lupus nephritis: a systematic review and metaanalysis. J Rheumatol. 2011 Jan;38(1):69-78. doi: 10.3899/jrheum.100130. Epub 2010 Oct 15. Review. PubMed PMID: 20952473.
3 Walsh M, James M, Jayne D, Tonelli M, Manns BJ, Hemmelgarn BR. Mycophenolate mofetil for induction therapy of lupus nephritis: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2007 Sep;2(5):968-75. Epub 2007 Aug 8. Review. PubMed PMID: 17702723.
An RCT demonstrated that the probability of remission of proteinuria in class V lupus nephritis over 12 months was significantly higher with cyclophosphamide and glucocorticoids compared to glucocorticoids alone.1 Cyclosporine showed a comparable rate of remission of proteinuria to cyclophosphamide; however, it was associated with a higher relapse rate. Radhakrishnan et al. published a study combining results from all patients with pure class V lupus nephritis from 2 RCTs comparing MMF (2-3g/d in divided doses) with IV cyclophosphamide as induction therapy for various classes of lupus nephritis.2 Both groups received concomitant glucocorticoids. The study revealed a significant change in mean proteinuria after 24 weeks of therapy in both groups, with stable serum creatinine levels. No significant differences were noted between the groups. Given the fertility concerns with cyclophosphamide, MMF is typically recommended over cyclophosphamide.
This evidence was graded as moderate quality as it used a nonspecified subgroup analysis of the RCT data.
This recommendation was rated as strong as there were good outcomes noted in the study, with stability of creatinine levels and reduction of proteinuria in a relatively high-risk subset of patients with class V lupus nephritis.
1 Austin HA 3rd, Illei GG, Braun MJ, Balow JE. Randomized, controlled trial of prednisone, cyclophosphamide, and cyclosporine in lupus membranous nephropathy. J Am Soc Nephrol. 2009 Apr;20(4):901-11. doi: 10.1681/ASN.2008060665. Epub 2009 Mar 18. PubMed PMID: 19297556; PubMed Central PMCID: PMC2663831.
2 Radhakrishnan J, Moutzouris DA, Ginzler EM, Solomons N, Siempos II, Appel GB. Mycophenolate mofetil and intravenous cyclophosphamide are similar as induction therapy for class V lupus nephritis. Kidney Int. 2010 Jan;77(2):152-60. doi: 10.1038/ki.2009.412. Epub 2009 Nov 4. PubMed PMID: 19890271.
A meta-analysis including 2 well-designed RCTs did not show a statistically significant difference between disease activity scores or risk of flares in patients taking oral contraceptive pills.1 The patient population was predominantly patients with SLE with well-controlled disease, and data cannot be confidently extrapolated to patients with active disease, nor to patients with antiphospholipid antibody positivity.
The evidence was graded as high-quality as it stems from 2 well-designed RCTs. It was given a strong recommendation due to the high-quality evidence and the ubiquitous use of estrogen-containing oral contraceptive pills in family planning.
1 Rojas-Villarraga A, Torres-Gonzalez JV, Ruiz-Sternberg ÁM. Safety of hormonal replacement therapy and oral contraceptives in systemic lupus erythematosus: a systematic review and meta-analysis. PLoS One. 2014 Aug 19;9(8):e104303. doi: 10.1371/journal.pone.0104303. eCollection 2014. Review. PubMed PMID: 25137236; PubMed Central PMCID: PMC4138076.
A cohort study has shown a significant association between oral contraceptive use in patients with APLAs and the risk of thrombosis.1 A meta-analysis in patients with SLE did not show an association between oral contraceptive use and thrombosis; however, notably in most of these studies, patients with APLAs were excluded.2
This evidence was graded as low-quality given the observational nature of the studies.
Despite the paucity of high-quality evidence to guide recommendations in this area, given the risk of potential morbidity in patients experiencing thrombosis as well as the availability of nonhormonal contraceptives, the authors give a strong recommendation to avoid estrogen-containing contraceptives in patients with SLE and APLAs.
1 Choojitarom K, Verasertniyom O, Totemchokchyakarn K, Nantiruj K, Sumethkul V, Janwityanujit S. Lupus nephritis and Raynaud's phenomenon are significant risk factors for vascular thrombosis in SLE patients with positive antiphospholipid antibodies. Clin Rheumatol. 2008 Mar;27(3):345-51. Epub 2007 Sep 2. PubMed PMID: 17805483.
2 Rojas-Villarraga A, Torres-Gonzalez JV, Ruiz-Sternberg ÁM. Safety of hormonal replacement therapy and oral contraceptives in systemic lupus erythematosus: a systematic review and meta-analysis. PLoS One. 2014 Aug 19;9(8):e104303. doi: 10.1371/journal.pone.0104303. eCollection 2014. Review. PubMed PMID: 25137236; PubMed Central PMCID: PMC4138076.
The available data suggest worse outcomes for patients with active disease at the time of conception and during pregnancy. A meta-analysis of maternal and pregnancy outcomes in patients with active or previous lupus nephritis have shown an increased risk of preterm birth and maternal hypertension in patients who had active disease at conception.1 A case-control study demonstrated an association between serological disease activity at the time of conception (hypocomplementemia and/or elevated dsDNA levels) and flares of lupus in later pregnancy.2 In this study, patients who had lupus flares in pregnancy had a higher risk of adverse outcomes, including eclampsia/preeclampsia, fetal loss, and preterm birth, compared to patients with stable inactive disease or low disease activity, and patients with active disease (both flares and newly-diagnosed disease) had a higher risk of maternal mortality and significant organ compromise. A retrospective review of 183 pregnancies in patients with SLE found that quiescent disease for >4 months prior to conception resulted in lower incidence rates of preterm birth (sensitivity, 71.4%; specificity, 61.5%), pregnancy loss (sensitivity, 70.8%; specificity, 53.2%), intrauterine growth restriction, and pregnancy-induced hypertension (sensitivity, 63.6%; specificity, 59.8%).3
This evidence was rated as low-quality due to the observational nature of the studies. However, given the potential maternal and fetal risks associated with active SLE in pregnancy, measures to control active SLE prior to conception were felt to merit a strong recommendation.
1 Smyth A, Oliveira GH, Lahr BD, Bailey KR, Norby SM, Garovic VD. A systematic review and meta-analysis of pregnancy outcomes in patients with systemic lupus erythematosus and lupus nephritis. Clin J Am Soc Nephrol. 2010 Nov;5(11):2060-8. doi: 10.2215/CJN.00240110. Epub 2010 Aug 5. Review. PubMed PMID: 20688887; PubMed Central PMCID: PMC3001786.
2 Yang H, Liu H, Xu D, et al. Pregnancy-related systemic lupus erythematosus: clinical features, outcome and risk factors of disease flares--a case control study. PLoS One. 2014 Aug 13;9(8):e104375. doi: 10.1371/journal.pone.0104375. eCollection 2014. PubMed PMID: 25118692; PubMed Central PMCID: PMC4131906.
3 Ko HS, Ahn HY, Jang DG, et al. Pregnancy outcomes and appropriate timing of pregnancy in 183 pregnancies in Korean patients with SLE. Int J Med Sci. 2011;8(7):577-83. Epub 2011 Oct 1. PubMed PMID: 22022210; PubMed Central PMCID: PMC3198253.