Global Initiative for Asthma. Global strategy for asthma management and prevention. Updated 2021. Accessed January 10, 2022. Available from www.ginasthma.org
Chung KF, Wenzel SE, Brozek JL, et al: International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014 Feb;43(2):343-73. doi: 10.1183/09031936.00202013. Epub 2013 Dec 12.
Definition, Etiology, PathogenesisTop
Asthma is a heterogeneous disease characterized by chronic airway inflammation. It is defined by the history of respiratory symptoms such as wheeze, shortness of breath, chest tightness, and cough that vary over time and in intensity, together with variable airflow limitation (Global Initiative for Asthma [GINA] 2021). Chronic inflammation is associated with airway hyperresponsiveness and recurrent symptoms that are often worse at night or early in the morning. Variable airflow limitation is caused by bronchial smooth muscle contraction, mucosal edema, and formation of “mucus plugs.” In some patients irreversible airflow limitation may be caused by airway remodeling.
There are several different asthma phenotypes, the most common of which is allergic asthma. In such cases the binding of an allergen to which the patient is sensitized to specific IgE antibodies on the surface of mast cells leads to the release of mediators (including histamine, cysteinyl leukotrienes, prostaglandin D2, and proteolytic enzymes), which cause airway obstruction. In some patients this early phase of allergic reaction is followed within 6 to 8 hours by a late-phase reaction in which mast cells, basophils, and other cells release cytokines and chemokines. This causes an increase in the influx of inflammatory cells, particularly eosinophils, to the airways. However, not all eosinophilic asthma can be shown to be associated with allergen sensitization. A preferred term for this phenotype is type 2 asthma because the cytokines that cause the persistent airway inflammation originate from a subpopulation of helper lymphocytes T helper 2 (Th-2) cells or innate lymphoid cells type 2. These cells produce a characteristic cytokine profile (interleukin 4 [IL-4], IL-5, IL-13), which influences the production of IgE by B cells and the growth, differentiation, and activation of eosinophils and mast cells. This subset of asthmatic patients has type 2–high (T2-high) airway inflammation.
Allergic asthma most often begins in childhood; may coexist with other atopic diseases; and is associated with family history of atopic diseases, positive prick skin tests to inhaled allergens, and allergen-specific IgE antibodies in blood. Patients with allergic asthma often have airway eosinophilia and good response to inhaled corticosteroids (ICSs).
Not all asthma patients have T2-high inflammation. The pathobiology of T2-low asthma is not as well understood. It may be triggered by an immune process initiated by a viral or bacterial infection, but the airway inflammatory cells are either neutrophils or are very scarce (paucigranulocytic asthma). Airway histopathology is similar as in allergic asthma.
Factors triggering asthma symptoms and exacerbations or prolonging their course: Allergens, respiratory infections (predominantly of viral etiology), air pollutants (including tobacco smoke, household aerosols, paint fumes), exercise, stress, weather conditions, drugs (beta-blockers, nonsteroidal anti-inflammatory drugs [NSAIDs]), food additives.
Factors increasing the risk of asthma exacerbations: Uncontrolled asthma, excessive use of inhaled beta2-agonists (use of >1 canister of 200 doses per month is associated with increased risk of death), lack of ICS treatment, forced expiratory volume in 1 second (FEV1) <60% of predicted value, major psychological or socioeconomic problems, exposure to tobacco smoke, exposure to allergens (in allergic asthma), comorbidities, increased sputum or blood eosinophilia, pregnancy, previous intubation or treatment in the intensive care unit (ICU) due to asthma, ≥1 severe exacerbation in the previous 12 months, increased exhaled nitric oxide (FeNO) in ICS-treated patients.
Clinical Features and Natural HistoryTop
1. Symptoms are variable in nature and consist of breathlessness, chest tightness, wheezing, and cough. They resolve spontaneously or after treatment. Cough can be the sole symptom. In some patients symptoms of other allergic diseases, most frequently allergic rhinitis, may coexist.
2. Signs: Wheezing and rhonchi (diffuse, bilateral, mainly expiratory) and a prolonged expiratory phase. These are sometimes observed only during forced expiration and may be absent in very severe exacerbations (silent chest). Signs and symptoms of asthma are often absent in the periods between attacks or exacerbations.
3. Natural history: Asthma may occur at any age, but it most commonly develops in childhood; in such cases it is usually allergic. Asthma with onset in adulthood is more frequently nonallergic and often has a more severe course. Asthma exacerbations are potentially severe and life-threatening events, which usually develop over several days.
DiagnosisTop
Diagnosis of asthma requires the presence of characteristic symptoms and demonstration of variable airflow obstruction. This can be done by using:
1. Spirometry, the most useful and widely used test to measure airflow obstruction. A diagnosis is established by a reduction in FEV1 with reversibility after inhaled beta2-agonist (an improvement in FEV1 >12% and >200 mL); however, normal spirometry results do not exclude the diagnosis of asthma.
2. Peak expiratory flow (PEF), a less precise method for measuring airflow obstruction. The measurements are usually made twice daily for several weeks. Mean diurnal PEF variability >10% (diurnal PEFmax − PEFmin/PEFmean, averaged for 2 weeks) identifies asthma. Short-term PEF monitoring can be used for assessing response to treatment, identifying factors triggering asthma attacks (eg, occupational factors), and establishing a baseline for action plans. Long-term monitoring is used in severe asthma or in patients with poor perception of symptoms.
3. Bronchial challenge tests, most often used to establish a diagnosis when spirometry is normal. The most widely used test is methacholine inhalation challenge. Other options are histamine or mannitol challenge. A positive bronchial challenge is not specific for asthma, but a negative test result helps exclude asthma; sometimes indirect airway challenges such as exercise or isocapnic hyperventilation are used.
1. Chest radiographs are usually normal. During an asthma exacerbation radiographs may reveal features of hyperinflation and certain complications of asthma exacerbations (eg, pneumothorax).
2. Pulse oximetry and (in acute severe exacerbations) arterial blood gas measurements may be performed to evaluate the severity of respiratory failure during exacerbations and to monitor their course. Notably, alveolar hyperventilation is usual in an acute severe exacerbation, so normal arterial partial pressure of carbon dioxide (arterial pCO2) may be a sign of impending respiratory failure. Venous blood gases may also allow evaluation and follow-up of pH and pCO2.
3. IgE-dependent allergic tests: Skin prick tests as well as total and specific serum IgE levels may identify allergens causing sensitization in patients with allergic asthma.
4. Measurement of FeNO levels is usually not recommended for the diagnosis of asthma, but FeNO is increased (>25 ppb) in steroid-naive persons with eosinophilic inflammation and may prove helpful for disease monitoring in assessing adherence to ICSs.
5. Sputum examination: Sputum eosinophilia is a marker of eosinophil inflammation in the airways and can be useful in guiding management (see below).
Asthma symptoms are nonspecific. The diagnosis, based on history and symptoms, must include demonstration of expiratory airflow limitation and variability in airflow.
Domains contributing to diagnosis are:
1) Presence of variable respiratory symptoms (wheezes, dyspnea, chest tightness, cough, heavy breathing); often worse at night or on waking; worsening with viral infections; sometimes triggered by allergens, cold air, exercise, environmental pollutants.
2) Documented airflow limitation and its variability:
a) >10% diurnal PEF variability (the greater the variability, the more likely the diagnosis of asthma).
b) Documented bronchodilator reversibility testing: An increase in FEV1 after 2 to 4 puffs of an inhaled beta2-agonist of >12% and >200 mL from baseline.
c) Improved airflow after 4 weeks of anti-inflammatory treatment by an increase in FEV1 by >12% and >200 mL or increase in PEF by 20%.
d) Bronchoconstriction after exercise challenge testing (FEV1 decrease by >10% and >200 mL from baseline); methacholine or histamine challenge test (FEV1 decrease from baseline by ≥20%); or mannitol, hypertonic saline, or hyperventilation challenge test (FEV1 decrease ≥15%).
e) Excessive variations in FEV1 between visits (>12% and >200 mL).
Classification of asthma control: Asthma control is determined by an assessment that covers the preceding 4 weeks:
1) Well-controlled asthma (all of the following criteria are fulfilled):
a) Daytime symptoms ≤2 times a week.
b) No nocturnal symptoms.
c) Reliever medications needed ≤2 times a week.
d) No activity limitation due to asthma.
2) Partly controlled asthma: 2 or 3 of the above criteria fulfilled.
3) Uncontrolled asthma: 0 or 1 of the above criteria fulfilled.
The future risk of exacerbations and fixed airflow limitation (see Definition, Etiology, Pathogenesis, above) as well as treatment issues (correct inhaler technique, adherence, adverse effects) should also be assessed. When the treatment level needed for asthma control is established (Figure 1), it is used in the evaluation of the severity of asthma, which may be mild (controlled with treatment step 1 or 2), moderate (controlled with step 3 treatment), or severe (treatment step 4 or 5 necessary).
Differential diagnosis includes chronic obstructive pulmonary disease (COPD), vocal cord dysfunction, hyperventilation with panic attacks, heart failure, bronchiectasis, pulmonary embolism, and respiratory tract infections.
Less frequent conditions include tumors of the respiratory tract, foreign body aspiration, tracheal stenosis after tracheostomy, bronchiolitis obliterans, acquired tracheobronchomalacia, hypereosinophilic syndromes. Also see Cough, see Dyspnea.
Distinguishing between asthma and COPD may occasionally be difficult and even lead to a diagnosis of asthma-COPD syndrome. Features favoring asthma include:
1) Younger age of onset.
2) Short-term variations in symptoms (over hours or days).
3) Symptoms worsening at night and early morning (as opposed to exertional).
4) Symptoms triggered in the short term by emotions, dust, allergens, or exercise (as opposed to chronic cough, sputum production, and dyspnea).
5) Variable versus persistent airflow limitation.
6) Lung function normal between symptoms (versus persistently abnormal).
7) General lack of progression of symptoms over time (seasonal variation likely) versus slow long-term progression.
8) Family history of asthma.
9) Lack of heavy exposure to COPD risk factors (mainly tobacco smoking).
10) Rapid response to bronchodilators and anti-inflammatory treatment (hours to days or short weeks) versus transient relief with short-acting bronchodilators.
11) Normal chest radiographs.
TreatmentTop
Asthma cannot be cured, but with appropriate treatment it is usually possible to achieve and maintain good asthma control.
Education is a vital part of treatment and consists of informing the patient about the diagnosis and nature of the disease, available treatments, drug inhalation techniques, measures used to reduce exposure to the factors triggering asthma attacks and exacerbations, and monitoring of disease control. Patients should have a personal written asthma action plan covering controller treatment and measures to be taken in case of exacerbations.
Nonpharmacologic Interventions
1. Encourage the patient to engage in physical activity (inform about exercise-induced bronchoconstriction and methods of its prevention).
2. Actively help to discontinue tobacco smoking (counseling, pharmacotherapy; see Nicotine Addiction).
3. Identify subjects with occupational exposures and provide counseling on the appropriate methods of prevention.
4. Recommend allergen avoidance methods in sensitized patients (for most indoor allergens these methods are of unproven effectiveness).
5. Schedule psychological counseling or mental health assessment when necessary.
6. Recommend annual flu vaccination.
1. General principles of pharmacotherapy: The following classes of asthma medications are used:
1) Controller medications, used on a regular basis include ICSs, inhaled long-acting beta2-agonists (LABAs), long-acting muscarinic antagonists (LAMAs), and leukotriene modifiers.
2) Reliever medications, used as needed, include rapid-onset LABA (formoterol) together with an ICS, taken as a reliever (ICS/formoterol) from a single device (the preferred reliever), inhaled rapid-onset beta2-agonists, inhaled anticholinergics.
3) Treatments used in severe asthma include oral corticosteroids (OCS), monoclonal antibodies directed against IgE (omalizumab), interleukin 5 (IL-5) (mepolizumab or reslizumab), IL-5Rα (benralizumab), IL-4Rα (dupilumab), and bronchial thermoplasty.
The choice of asthma medications depends on the level of asthma control and previous treatment. Currently 2 pathways of pharmacotherapy are suggested, based on the choice of reliever:
1) The preferred reliever is ICS/formoterol (used regularly in more severe asthma).
2) Another choice is SABA used as a reliever, with regular anti-inflammatory treatment (treatment steps: Figure 16.2-1).
In patients with infrequent symptoms (≤2 times a month, without night symptoms), normal lung function, and no risk factors for exacerbations, only intermittent treatment with a reliever is suggested (step 1). Previously short-acting beta2-agonist (SABA) was the reliever of choice for patients with intermittent symptoms. However, the combination of ICS/formoterol from the same device, used as a reliever, has been shown to be more effective at improving asthma control and reducing the risk of asthma exacerbations when compared with SABA as a reliever.Evidence 1Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of the intervention). O'Byrne PM, FitzGerald JM, Bateman ED, et al. Inhaled Combined Budesonide-Formoterol as Needed in Mild Asthma. N Engl J Med. 2018 May 17;378(20):1865-1876. doi: 10.1056/NEJMoa1715274. PubMed PMID: 29768149. Bateman ED, Reddel HK, O'Byrne PM, et al. As-Needed Budesonide-Formoterol versus Maintenance Budesonide in Mild Asthma. N Engl J Med. 2018 May 17;378(20):1877-1887. doi: 10.1056/NEJMoa1715275. PubMed PMID: 29768147.
In previously untreated patients with more frequent symptoms of asthma, patients can be offered a choice of treatment plans for step 2 treatment. GINA guidelines prefer continuing the combination of ICS/formoterol used only as a reliever. This treatment approach provides slightly less asthma control but is equally effective at reducing exacerbation risk as maintaining low-dose ICS therapy (which is the other option in these patients) and at a lower daily ICS dose.Evidence 2Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of the intervention). O'Byrne PM, FitzGerald JM, Bateman ED, et al. Inhaled Combined Budesonide-Formoterol as Needed in Mild Asthma. N Engl J Med. 2018 May 17;378(20):1865-1876. doi: 10.1056/NEJMoa1715274. PubMed PMID: 29768149. Bateman ED, Reddel HK, O'Byrne PM, et al. As-Needed Budesonide-Formoterol versus Maintenance Budesonide in Mild Asthma. N Engl J Med. 2018 May 17;378(20):1877-1887. doi: 10.1056/NEJMoa1715275. PubMed PMID: 29768147. The main problem with the latter approach is very poor patient adherence to daily use of ICS.
Consider step 3 treatment in those with symptoms on most days or night symptoms ≥1 time per week. The preferred options are regular plus as needed low-dose ICS/formoterol or low-dose ICS plus another LABA. Using a higher dose of ICS or adding an LTRA or house dust mite (HDM) sublingual immunotherapy (SLIT) are other options (Figure). Controller medications result in clinical improvement within a few days from the beginning of treatment and their full therapeutic effects develop in 1 to 2 months. Usually first repeat visit should take place after 1 to 3 months, and subsequently after 3 months. Frequent use of reliever medications (≥2 times/wk) is an indicator of poor asthma control and need for intensification of controller treatment.
Patients who do not achieve asthma control with step 3 treatment need to be reevaluated for other conditions or factors responsible for severe asthma (see below).
In patients maintaining adequate asthma control for ≥3 months, consider a step-down in treatment intensity, depending on treatment that achieved adequate asthma control:
1) ICS: Reduce the dose by 50% or (when a low dose was used) to once daily or to as-needed low-dose ICS/formoterol.
2) ICS + LABA: Reduce the ICS dose by 50% or to once daily (when a low dose was used) and continue LABA. LABA discontinuation is more likely to lead to deterioration.
3) ICS + formoterol (as maintenance and reliever treatment): Reduce the ICS dose (by 50% or to a low dose), continue maintenance and as-needed treatment.
4) ICS + second controller: Reduce the ICS dose by 50% and continue the second controller.
5) High-dose ICS + LABA + OCS: Reduce the OCS dose in a stepwise manner, then administer it every other day. A sputum-guided approach may be used in experienced centers.
In patients in whom adequate asthma control could not be achieved with step 3 treatment, reassess the patient for other diseases and causes of severe refractory asthma.
2. Controller medications (administered on a regular basis):
1) ICS: ICSs are the most effective and preferred asthma controller medications and should be the first choice for therapy.Evidence 3 Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of the intervention). Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012 May 16;5:CD002314. doi:10.1002/14651858.CD002314.pub3. Review. PubMed PMID: 22592685; PubMed Central PMCID: PMC4164381. Dosage: Table 1. Local adverse effects include oral candidiasis, hoarseness, and cough due to throat irritation. Prevention of adverse effects: mouth rinsing after drug inhalation (use of spacers when administering drugs via a metered-dose inhaler [MDI]) or use of prodrugs (eg, ciclesonide). Long-term high-dose treatment may cause systemic adverse effects (see Cushing Syndrome).
2) LABA: Table 1. It is recommended that a regular LABA never be used without concomitant ICS therapy.Evidence 4 Strong recommendation against (downsides clearly outweigh benefits; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of the intervention). Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM; SMART Study Group. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006 Jan;129(1):15-26. Erratum in: Chest. 2006 May;129(5):1393. PubMed PMID: 16424409. Cates CJ, Wieland LS, Oleszczuk M, Kew KM. Safety of regular formoterol or salmeterol in adults with asthma: an overview of Cochrane reviews. Cochrane Database Syst Rev. 2014 Feb 6;2:CD010314. doi: 10.1002/14651858.CD010314.pub2. Review. PubMed PMID: 24504983. To prevent the use of LABA alone, the agents should be prescribed as a fixed combination inhaler containing LABA and ICS. Fixed-combination inhalers containing formoterol and ICS can be used as needed only or regularly and as needed. When used also as a reliever, the maximal daily dose of formoterol should not exceed 72 microg (when used with budesonide) or 48 microg (when used with beclomethasone). The most frequent adverse effects of LABAs include tachycardia, muscle tremor, and hypokalemia.
3) Leukotriene modifiers: Montelukast 10 mg once daily, zafirlukast 20 mg bid. These drugs are less effective than ICS monotherapy. The United States Food and Drug Administration (FDA) warns about serious mental health adverse effects (including suicidal thoughts or actions) associated with montelukast use.
4) Long-acting muscarinic antagonists: In patients with lack of asthma control despite using a medium-dose or high-dose ICS + LABA treatment, tiotropium via a soft-mist inhaler 5 microg once daily can be added or treatment switched to triple therapy inhaler (LABA + LAMA + ICS).
5) Sustained-release theophylline is no longer recommended for asthma, as it is less effective than inhaled drugs and more frequently causes significant adverse effects.
3. Reliever medications (administered as needed): Rapid-onset short-acting inhaled beta2-agonists (fenoterol, albuterol, terbutaline) (Table 1) are used solely for asthma symptom relief or to prevent exercise-induced bronchoconstriction; they cause rapid symptomatic relief. The onset of action is after a few minutes, peak effect develops after ~15 minutes, and effect lasts for 4 to 6 hours. A fixed combination of ICS/formoterol in one inhaler is the preferred reliever medication in step 1 and step 2 patients and can be used both as a controller and a reliever medication for at least step 3 patients.
4. Treatment used in severe asthma:
1) OCS: Prednisone, prednisolone, methylprednisolone. Used only for exacerbations and in patients with severe refractory asthma (step 5 treatment) because of serious adverse effects (see Cushing Syndrome). Discuss the treatment decision with the patients, as they should be informed about the benefits and risks of therapy. For intensification of long-term controller treatment, usually 20 to 30 mg once daily in the morning is used; then taper off to the lowest dose providing good asthma control (this may be as low as 5 mg/d). Long-term treatment with OCS warrants prevention of osteoporosis (see Osteoporosis).
2) Monoclonal antibodies are used in patients with moderate or severe allergic or eosinophilic asthma that is not controlled with step 4 treatment (Table 2). In appropriately selected patients these treatments have been shown to reduce severe exacerbation risk and may improve lung function. Evaluate the effectiveness of treatment after 4 to 6 months.
3) Bronchial thermoplasty: Consider this in very carefully selected patients with severe refractory noneosinophilic asthma.
5. Allergen-specific immunotherapy: Consider HDM SLIT for sensitized patients with asthma and allergic rhinitis and FEV1 >70%. Subcutaneous immunotherapy is effective but rarely used due to risk of serious adverse effects (including anaphylaxis) and inconvenience for the patient.
1. Management depends on the severity of exacerbation. Assessment and treatment in primary care: Figure 2. In mild exacerbations well-educated patients can modify treatment by:
1) Increasing the frequency of reliever inhalations.
2) Increasing the dose of ICS 2 to 4 times (unnecessary when the patient is using the ICS/formoterol combination as a reliever).
2. Goals of treatment to be achieved as soon as possible:
1) Relieve airway obstruction using inhaled rapid-onset beta2-agonists.
2) Relieve hypoxemia using oxygen therapy (see Oxygen Therapy).
3) Reduce inflammation and prevent recurrent exacerbations by early administration of systemic corticosteroids.
3. Treatment monitoring should be continuous or frequently repeated and include:
1) Evaluation of the severity of signs and symptoms and response to treatment.
2) Spirometry or PEF (if possible, measure the baseline value before initiation of treatment but only if this does not delay therapy; then repeat PEF until an evident response to treatment is achieved).
3) Respiratory rate.
4) Heart rate.
5) Measurement of hemoglobin oxygen saturation in arterial blood (pulse oximetry [SpO2]); blood gases in patients with life-threatening asthma attacks or with SpO2 <90%.
Patients at high risk of fatal outcomes of asthma include those with a history of life-threatening asthma exacerbations requiring mechanical ventilation; patients who were hospitalized or needed urgent medical intervention due to asthma in the preceding year; patients who currently use or have recently discontinued OCS; patients who do not currently take ICSs; patients who have required frequent as-needed inhalations of beta2-agonists; and patients with a psychiatric disorder or history of psychosocial problems.
1. Inhaled albuterol (Table 1):
1) Via an MDI with a spacer: 4 to 10 doses of 100 microg every 20 minutes in mild and moderate exacerbations, up to 20 doses within 10 to 20 minutes in severe exacerbations. Later 2 to 6 doses every 3 or 4 hours in mild exacerbations, 6 to 10 doses every 1 to 2 hours in moderate exacerbations. In severe exacerbations more doses may be necessary.
2) Via a nebulizer (preferably oxygen-driven): This is usually not required except if the patient is not responding to commands. Doses of albuterol are 2.5 to 5 mg every 15 to 20 minutes, continuous nebulization 10 mg/h in severe exacerbations.
In very exceptional cases of patients in whom inhaled drug administration is impossible, use IV albuterol. Dosage: 4 microg/kg over 10 minutes, then a continuous infusion 0.1 to 0.2 microg/kg/min with continuous heart rate monitoring; alternatively administer 0.5 mg subcutaneously.
2. Oxygen should be administered as soon as possible in all patients with a severe asthma attack via nasal prongs or a mask (see Oxygen Therapy) to achieve SpO2 93% to 95% (partial pressure of oxygen in arterial blood [PaO2] ≥60 mm Hg).
3. Systemic corticosteroids are used in all asthma exacerbations (except for the mildest cases), usually for 5 to 7 days. The first clinical effects are seen after 4 to 6 hours. Oral administration is as effective as the IV route if the patient can swallow the tablets and does not vomit (in such cases give an equivalent dose of IV corticosteroid). It is recommended to start a concomitant ICS as soon as the patient starts to improve. Dosage: oral prednisone, prednisolone, or methylprednisolone 1 mg/kg, up to 50 mg/d for 5 to 7 days, until satisfactory clinical improvement is achieved; IV methylprednisolone, dosage as above; IV hydrocortisone initial dose 100 to 200 mg, then 50 to 100 mg every 6 hours.
4. Other drugs:
1) Ipratropium bromide (Table 1) should be added to albuterol in patients with moderate to severe exacerbations:
a) Via an MDI: 4 to 8 doses of 20 microg repeated every 15 to 20 minutes; in severe exacerbation up to 20 doses within 10 to 20 minutes.
b) Nebulized: 0.25 to 0.5 mg repeated every 15 to 20 minutes or continuous nebulization (combined with albuterol).
2) IV magnesium sulfate may be used in severe exacerbations if the patient is not responding to frequent administration of inhaled albuterol. Dosage: 1 to 2 g infused over 20 minutes.
3) IV theophylline should not be used.
4) Antibiotics should only be used in case of bacterial respiratory tract infection.
Treatment of Respiratory Failure
See Acute Respiratory Failure.
Before discharging the patient from the hospital, make sure they have learned the correct inhaler technique and arrange a follow-up visit (usually after 2-7 days). Educate the patient and modify the written asthma action plan when appropriate. Increase the dose of ICS for 2 to 4 weeks.
Special ConsiderationsTop
1. During pregnancy both deterioration and improvement of asthma control may occur. Poorly controlled asthma and fetal hypoxia are more dangerous than potential adverse drug reactions from asthma medications. Patient education is very important to ensure these medications are not stopped by the pregnant woman.
2. The principles of asthma controller treatment and management of exacerbations in pregnant patients are the same as those used for the general patient population. Preferred medications include ICSs (or OCS if necessary, but efforts should be made to avoid these during the first trimester because of increased risk of cleft palate) and inhaled SABAs (data on the safety of LABAs is limited).
3. Women who were treated with prednisone in doses >7.5 mg/d for >2 weeks before delivery should receive IV hydrocortisone 50 to 100 mg every 6 to 8 hours during delivery. This is a pattern of practice based on a low quality of evidence.
4. Monitor blood glucose levels for 24 hours in the newborn if the mother received high doses of SABA during labor and delivery.
5. All asthma medications may be used in breastfeeding women.
1. Prior to surgery asthma control and pulmonary function should be evaluated. Optimally the measurements should be performed early enough to allow for necessary intensification of treatment.
2. Patients undergoing surgical interventions associated with major perioperative stress (except for minor surgery and surgical procedures in local anesthesia) who were treated with systemic corticosteroids in doses ≥20 mg/d of prednisone or equivalent doses of other corticosteroids for ≥2 weeks in the preceding 6 months should receive IV hydrocortisone in the perioperative period (up to 24 hours after surgery) 50 to 100 mg every 8 hours with the first dose administered before surgery. This is a pattern of practice based on a low quality of evidence.
Ensure the patient about the safety of inhaled drugs. If possible, avoid nebulization and spirometry in individuals with confirmed or suspected coronavirus disease 2019 (COVID-19).
1. Difficult-to-treat asthma (requires step 4-5 to maintain control or remains not fully controlled despite this therapy) and severe asthma (remains not fully controlled despite optimized therapy of maximal intensity). Management:
1) Confirm the diagnosis (see Differential Diagnosis, above).
2) Assess compliance (including proper inhalation technique).
3) Identify factors negatively influencing asthma control:
a) Comorbidities (rhinosinusitis, allergic bronchopulmonary aspergillosis, gastroesophageal reflux disease, obesity, obstructive sleep apnea syndrome, anxiety/depression).
b) Exposure to factors triggering asthma symptoms or exacerbations.
c) SABA overuse.
d) Adverse effects of medications.
e) Anxiety, depression, social problems.
4) Optimize treatment: Diagnose severe asthma if after 3 to 6 months the disease remains uncontrolled. Repeat steps 1 to 4 and consider multidisciplinary approach. Assess T2-high inflammation during high-dose ICS (or OCS) treatment, which is present if ≥1 of the following are met:
a) Blood eosinophils ≥300/microL.
b) Sputum eosinophils ≥2%.
c) FeNO ≥25 ppb.
d) Worsening of symptoms after exposure to an allergen.
If T2-high features are met:
a) Assess compliance with objective methods (based on pharmacy data, inhaler with an electronic dose counter or measuring the prednisone level in blood).
b) Consider increasing ICS dose for 3 to 6 months
c) Consider additional pharmacotherapy with a LAMA.
d) Consider biologic treatment (Table 2).
If T2-high features are not met:
a) Consider additional studies (induced sputum, high-resolution computed tomography [HRCT]).
b) Optimize additional pharmacotherapy considering LAMA, macrolide, and a low-dose OCS.
c) Consider bronchial thermoplasty.
2. Aspirin-induced asthma (AIA) or aspirin-exacerbated respiratory disease (AERD) is a specific type of asthma that occurs in ~10% of adult patients with asthma. AIA usually starts with persistent rhinitis, which leads to sinusitis and eventually to asthma. Nasal polyps and eosinophilia are frequent. Typical features of AIA are asthma attacks frequently associated with rhinorrhea, conjunctival irritation, and flushing of the head and neck developing within several minutes to hours following ingestion of aspirin (acetylsalicylic acid [ASA]) or other nonsteroidal anti-inflammatory drugs (NSAIDs). Analgesics safe for patients with AIA include acetaminophen (INN paracetamol) (single doses ≤1 g), salicylamide, and celecoxib. Despite avoidance of ASA and other NSAIDs, asthma persists and frequently has a severe course. The sole objective diagnostic method is aspirin challenge, which may only be performed in specialized centers that also provide desensitization.
3. Work-related asthma refers to occupational asthma (caused by conditions in the occupational environment) and work-exacerbated asthma (preexisting or concurrent asthma worsened by the occupational environment). Approximately 400 factors inducing occupational asthma or exacerbating asthma have been described. Two distinct types of occupational asthma have been identified:
1) Immunologic: Induced by allergens (IgE-dependent) or low-molecular-weight sensitizers (IgE-independent), most frequently develops insidiously, with variable latency periods. It is usually preceded by a complex of prodromal symptoms (eg, cough, rhinitis, or conjunctivitis).
2) Nonimmunologic: Called reactive airways dysfunction syndrome (RADS), induced by irritants, results from exposure to very high levels of chemical irritants and develops up to 24 hours after exposure without prodromal symptoms. It is characterized by severe long-standing nonspecific bronchial hyperresponsiveness.
Treatment is the same as in nonoccupational asthma. It is necessary to eliminate occupational exposure to the noxious factor. In some patients this leads to alleviation of symptoms and sometimes even to complete remission. Details: see Work-Related Asthma.
4. Exercise-induced bronchoconstriction: In patients with asthma bronchoconstriction can occur within 5 to 10 minutes after the end of exercise and resolves spontaneously in up to 30 to 45 minutes (it frequently develops in patients with poorly controlled asthma).
Diagnosis: An FEV1 decrease ≥10% during exercise challenge or surrogate challenge (hyperventilation, inhalation of hypertonic saline or mannitol).
Management: SABA 15 minutes before exercise. In patients using SABAs daily, introduce regular controller treatment: ICS (with or without LABA), leukotriene modifier, or both. An appropriate warm-up before exercise may alleviate the symptoms. Patients exercising in cold weather may consider wearing a mask to warm the inhaled air.
5. Asthma-COPD overlap (ACO): ACO is diagnosed when features of asthma (variability in symptoms/lung function) and COPD (persistent airflow limitation) are present. Treatment: Smoking cessation, pulmonary rehabilitation, ICS + LABA.
Tables and FiguresTop
|
Medication |
Forms |
Dosage |
|
SABAs | ||
|
Fenoterol |
MDI 100 microg |
Reliever: 1-2 doses |
|
Albuterol (INN salbutamol) |
MDI 100 microg |
Reliever: 1-2 doses |
|
Terbutaline |
DPI 500 microg |
Reliever: 1-2 doses |
|
LABAs | ||
|
Formoterol |
MDI 12 microg DPI 4.5, 9, and 12 microg |
1-2 doses bid (max, 54 microg/d) |
|
Salmeterol |
MDI 25 microg |
1-2 doses bid (max, 200 microg/d) |
|
ICSs | ||
|
Beclomethasone |
MDI 100 and 250 microg |
50-100 microg bid (low dose) 100-200 microg bid (medium dose) >200 microg bid (high dose) |
|
Budesonide |
MDI 200 microg |
100-200 microg bid (low dose) >200-400 microg bid (medium dose) >400 microg bid (high dose) |
|
Ciclesonide |
MDI 80 and 160 microg |
80-160 microg once daily (low dose) >160-320 microg once daily (medium dose) >320 microg once daily (high dose) |
|
Fluticasone (propionate) |
MDI 50, 125, and 250 microg |
50-125 microg bid (low dose) >125-250 microg bid (medium dose) >250 microg bid (high dose) |
|
Mometasone |
MDI 400 microg |
110-220 microg/d (low dose) >220-440 microg/d (medium dose) >440 microg/d (high dose) |
|
Fixed combination of LABA + ICS in one inhaler | ||
|
Formoterol + budesonide |
DPI 4.5 microg/80 microg, 4.5 microg/160 microg, 9 microg/320 microg |
1-2 doses bid |
|
Salmeterol + fluticasone propionate |
MDI 25 microg/50, 125, or 250 microg DPI 50 microg/100, 250, or 500 microg |
1-2 doses bid |
|
Formoterol + beclomethasone |
MDI 6 microg/100 microg |
1-2 doses bid |
|
Vilanterol + fluticasone furoate |
DPI 25 microg/100 microg, 25 microg/200 microg |
1 dose a day |
|
Formoterol + mometasone |
MDI 5 microg/50, 100, or 200 microg |
1-2 doses bid |
|
Anticholinergics | ||
|
Ipratropium (short-acting) |
MDI 20 microg Nebulizer solution (0.25 mg/mL) |
In exacerbations (see text) |
|
Tiotropium (long-acting) |
Soft mist inhaler 2.5 microg |
1-2 doses daily |
|
SABA + short-acting anticholinergic in 1 inhaler | ||
|
Ipratropium + fenoterol |
MDI 20 microg/50 microg |
In exacerbations (see text) |
|
Fixed combination of LABA + LAMA+ ICS in 1 inhaler | ||
|
Formoterol + glycopyrronium + beclomethasone |
MDI 6 microg/12.5 microg/100 microg |
2 doses bid |
|
bid, 2 times a day; DPI, dry powder inhaler; ICS, inhaled corticosteroid; INN, international nonproprietary name; LABA, long-acting beta2-agonist; LAMA, long-acting muscarinic antagonist; MDI, metered-dose inhaler; qid, 4 times a day; SABA, short-acting beta2-agonist; tid, 3 times a day. | ||
|
Mechanism of action |
Medication |
Dosage |
|
Anti-IgE monoclonal antibody |
Omalizumab |
75-600 mg SC (based on baseline serum IgE level), 1-4 injections every 2-4 weeks |
|
Anti-IL-5 monoclonal antibodies |
Mepolizumab |
100 mg SC every 4 weeks |
|
Reslizumab |
3 mg/kg IV every 4 weeks | |
|
Anti-IL-5 receptor monoclonal antibody |
Benralizumab |
30 mg SC every 4 weeks for 3 months, then every 8 weeks |
|
Anti-IL-4 receptor monoclonal antibody |
Dupilumab |
Dose 1: 400-600 mg; then 200 or 300 mg SC every 2 weeks |
|
IL, interleukin; IV, intravenous; SC, subcutaneous. | ||
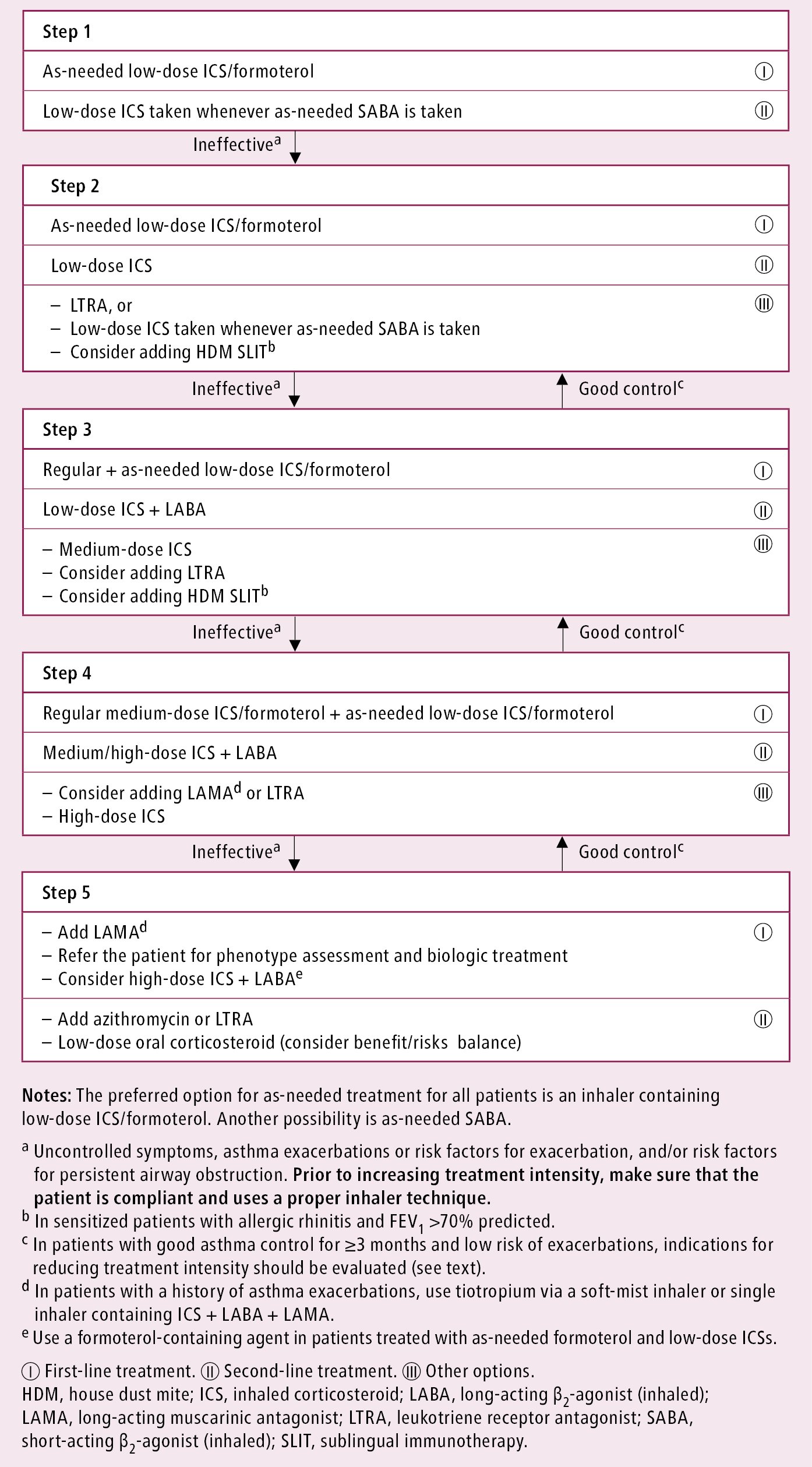
Figure 17.1-1. Controller treatment of asthma in adults. Based on the 2021 Global Initiative for Asthma (GINA) guidelines.
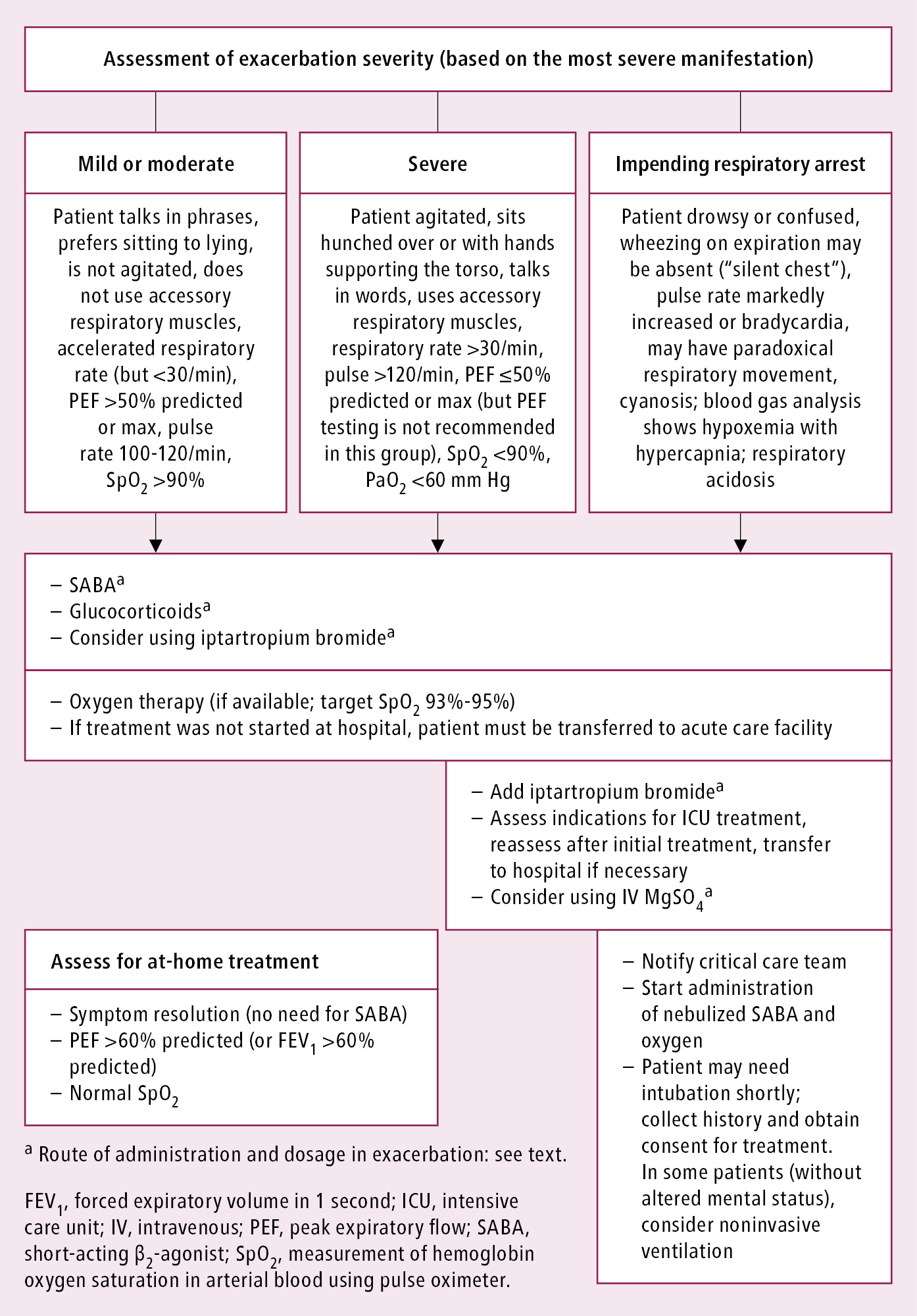
Figure 17.1-2. Management of asthma exacerbations depending on severity. Based on the 2021 Global Initiative for Asthma (GINA) guidelines.