Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid. 2017 Mar;27(3):315-389. doi: 10.1089/thy.2016.0457. Erratum in: Thyroid. 2017 Sep;27(9):1212. PubMed PMID: 28056690.
Ross DS, Burch HB, Cooper DS, et al. 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid. 2016 Oct;26(10):1343-1421. Erratum in: Thyroid. 2017 Nov;27(11):1462. PubMed PMID: 27521067.
Bartalena L, Baldeschi L, Boboridis K, et al; European Group on Graves' Orbitopathy (EUGOGO). The 2016 European Thyroid Association/European Group on Graves' Orbitopathy Guidelines for the Management of Graves' Orbitopathy. Eur Thyroid J. 2016 Mar;5(1):9-26. doi: 10.1159/000443828. Epub 2016 Mar 2. PubMed PMID: 27099835; PubMed Central PMCID: PMC4836120.
Definition, Etiology, Pathogenesis Top
A toxic thyroid nodule is an adenoma (toxic adenoma) or hyperplastic nodule observed on radionuclide scintigraphy that usually (but not always) causes hyperthyroidism. It develops most commonly as a result of a somatic mutation of the thyrotropin-stimulating hormone receptor or stimulatory G protein alpha subunit (Gs alpha). Unlike multinodular goiter, it is not related to iodine deficiency.
Clinical Features Top
Symptoms of hyperthyroidism are similar to those in toxic multinodular goiter and in thyrotoxicosis due to other causes. The typical pattern of development is from subclinical hyperthyroidism to overt hyperthyroidism (Figure 1). Also see Thyrotoxicosis and Hyperthyroidism.
Diagnosis Top
Diagnosis is based on radionuclide thyroid scintigraphy demonstrating one region of increased tracer activity with reduced or suppressed rest of the gland.
Clinical examination may reveal a solitary nodule. Thyroid-stimulating hormone (TSH) secretion can be decreased (but still within normal limits) or suppressed (subclinical or overt hyperthyroidism [see Thyrotoxicosis and Hyperthyroidism]). An autonomous (hot) nodule does not require fine-needle biopsy (FNB), considering its low risk of malignancy.
Treatment Top
1. Pharmacologic treatment: Thionamides (see Thyrotoxicosis and Hyperthyroidism) reduce symptoms of hyperthyroidism, but their discontinuation invariably results in the recurrence of hyperthyroidism. They may be used temporarily to help control hyperthyroidism in preparation for definitive treatment with radioiodine (RAI) therapy or surgery, particularly in the elderly population. Patients who do not want definitive treatment (see below) or have contraindications to RAI treatment or surgery can be treated with a long-term thionamide (preferably methimazole). Beta-blockers are used as in other cases of hyperthyroidism for symptom management.
2. RAI treatment is the treatment of choice for toxic adenoma. Due to low serum TSH levels, iodine uptake of the normal thyroid parenchyma is inhibited (therefore the risk of developing posttreatment hypothyroidism is low). RAI therapy should be planned for use after withholding of thionamides for 5 to 7 days (if used for hyperthyroid control prior to RAI therapy). Thionamides may be restarted 5 to 7 days after RAI treatment. TSH levels should be monitored periodically after the therapy. Temporary replacement therapy with levothyroxine may be necessary after RAI therapy until the previously suppressed normal thyroid gland recovers.
3. Surgical treatment is indicated for patients with an obstructive or very large nodule, coexisting malignancy or primary hyperthyroidism, contraindications to RAI therapy, or if it is the patient’s preference. For patients with toxic adenoma without nodules in the contralateral lobe, lobectomy is sufficient. For those with nonfunctioning nodules in the contralateral lobe, total thyroidectomy might be needed depending on FNB results, nodule characteristics on thyroid ultrasonography, or both. After total thyroidectomy, start thyroid replacement therapy and monitor TSH for dose adjustment. Serum calcium and parathyroid hormone (PTH) levels should be checked and followed given the risk of permanent or transient hypoparathyroidism.
Prognosis Top
The 6-year risk of developing overt hyperthyroidism in patients with subclinical thyrotoxicosis is 2% to 5%. With appropriately conducted RAI therapy, complete resolution of hyperthyroidism and achievement of euthyroidism is seen in most patients.
FiguresTop
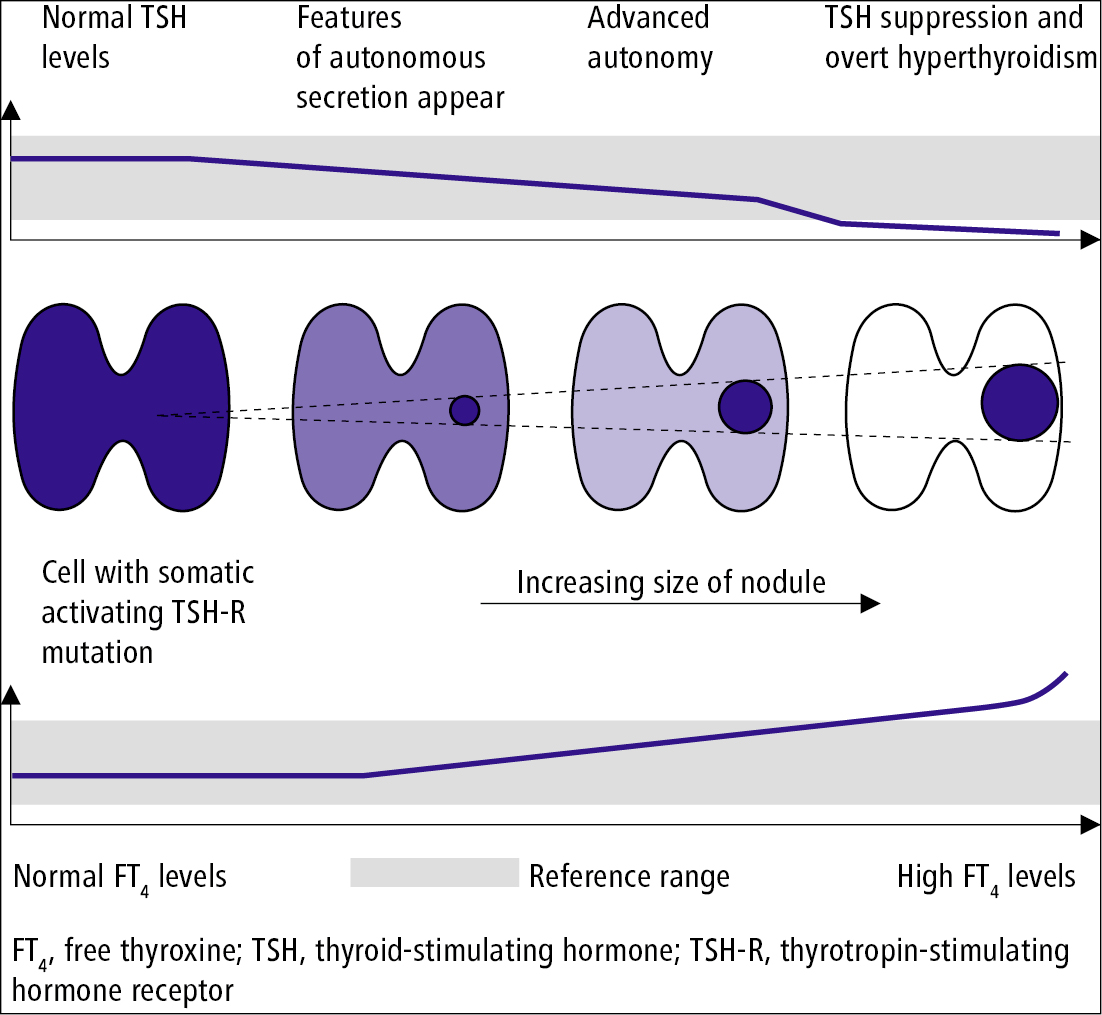
Figure 6.7-9. Development of an autonomously functioning thyroid nodule.