Mutaciones en los genes que regulan los procesos epigenéticos en la mastocitosis
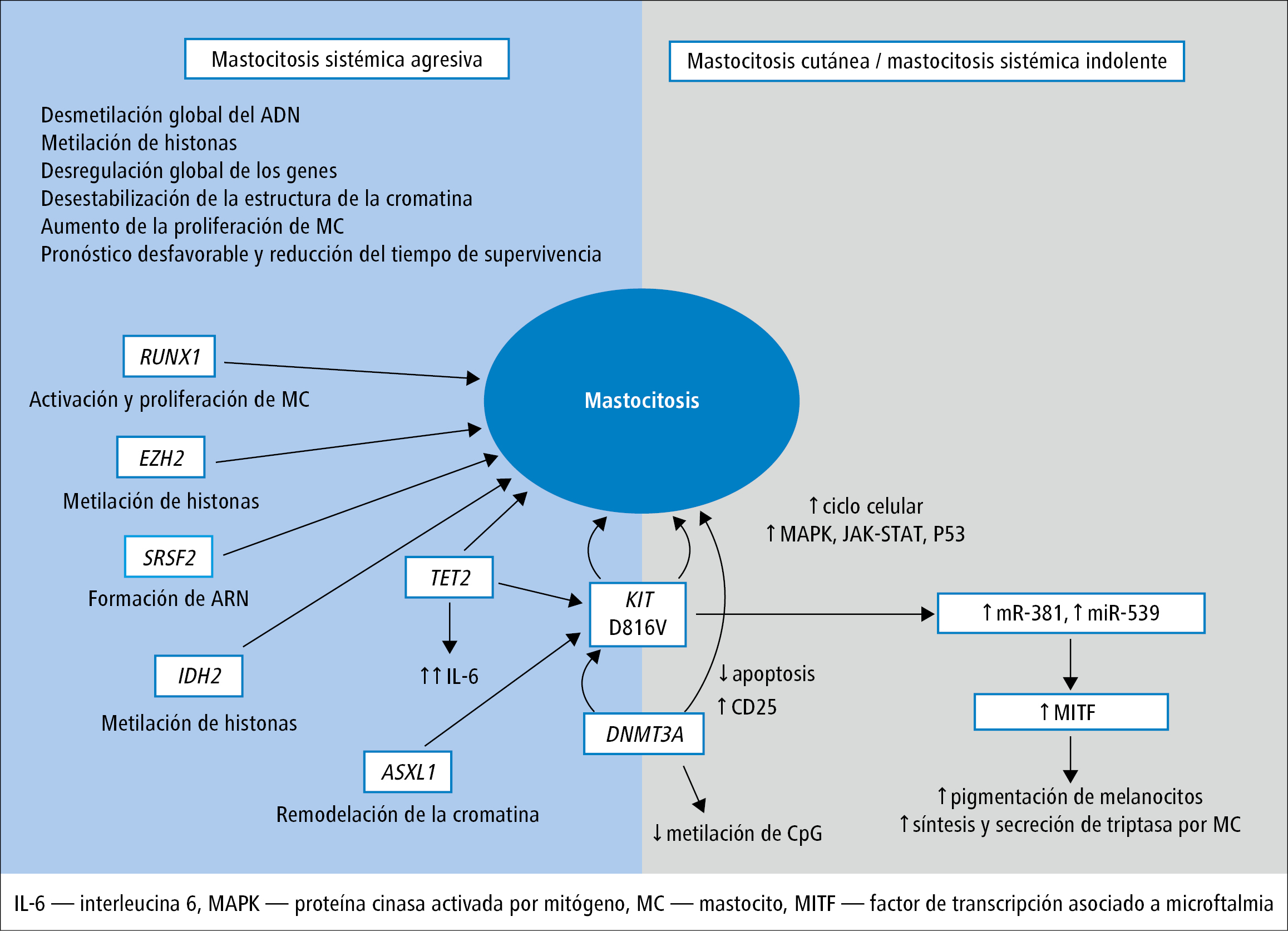
Las anomalías en la regulación de los mecanismos epigenéticos de la expresión génica pueden influir significativamente en el desarrollo de la mastocitosis por medio de los siguientes fenómenos: expresión de micro-ARN específicos, pérdida de función de genes supresores, activación de oncogenes (tirosinas cinasas, proteínas de las vías de señalización), defectos de replicación y reparación del ADN, regulación de la apoptosis e inestabilidad del genoma.40-42 En los mastocitos con mutación del gen KIT se observa una expresión anómala de micro-ARN. Lee y cols. observaron una menor expresión de miR 539 y miR 381 en estas células. Estos micro-ARN participan en la inhibición de la expresión del MITF (microphthalmiaassociated transcription factor), que regula el desarrollo de los mastocitos y los melanocitos, así como la síntesis de triptasa y melanina (fig. 5).40-42
Los estudios con secuenciación multigénica sobre las neoplasias del sistema hematopoyético han revelado numerosas mutaciones que suelen producirse en la mastocitosis.43-47 Estas mutaciones afectan a los genes que regulan el proceso de formación del ARNm, la transmisión de señales y los mecanismos epigenéticos. Las mutaciones de los genes que participan en los mecanismos epigenéticos son frecuentes en las formas avanzadas de mastocitosis. Están ligadas a un pronóstico desfavorable y un menor tiempo de supervivencia. Por lo general, son las siguientes mutaciones: TET2 (desmetilación DNA), DNMT3A (metilación de islas CpG), ASXL1 (remodelación de la cromatina) y IDH2 (regulación de la metilación de histonas) (fig. 5).43-47
Fig. 5. Mutaciones y cambios epigenéticos más importantes en la mastocitosis
Bibliografía:
1. Elieh Ali Komi D., Wöhrl S., Bielory L., Mast cell biology at molecular level: a comprehensive review, Clin. Rev. Allergy Immunol., 2020; 58: 342-3652. Valent P., Akin C., Bonadonna P. y cols., Proposed diagnostic algorithm for patients with suspected mast cell activation syndrome, J. Allergy Clin. Immunol. Pract., 2019; 7: 1125-1133
3. Shimbori C., Upagupta C., Bellaye P.-S. y cols., Mechanical stress-induced mast cell degranulation activates TGF-beta1 signalling pathway in pulmonary fibrosis, Thorax, 2019; 74: 455-465
4. Gorska A., Niedoszytko M., Lange M. y cols., Risk factors for anaphylaxis in patients with mastocytosis, Pol. Arch. Med. Wewn., 2015; 125: 46-53
5. Valent P., Akin C., Gleixner K.V. y cols., Multidisciplinary challenges in mastocytosis and how to address with personalized medicine approaches, Int. J. Mol. Sci., 2019; 20: 2976
6. Valent P., Akin C., Arock M. y cols., Definitions, criteria and global classification of mast cell disorders with special reference to mast cell activation syndromes: a consensus proposal, Int. Arch. Allergy Immunol., 2012; 157: 215-225
7. Valent P., Akin C., Escribano L. y cols., Standards and standardization in mastocytosis: consensus statements on diagnostics, treatment recommendations and response criteria, Eur. J. Clin. Invest., 2007; 37: 435-453
8. Akin C., Valent P., Metcalfe D.D., Mast cell activation syndrome: proposed diagnostic criteria, J. Allergy Clin. Immunol., 2010; 126: 1099-1104
9. Gell P.G.H. y cols., Clinical aspects of immunology, 1st ed. Oxford, England: Blackwell 1963
10. Metcalfe D.D., Mast cells and mastocytosis, Blood, 2008; 112: 946-956
11. Kulka M., Alexopoulou L., Flavell R.A., Metcalfe D.D., Activation of mast cells by double-stranded RNA: evidence for activation through Toll-like receptor 3, J. Allergy Clin. Immunol., 2004; 114: 174-182
12. Niedoszytko M., Bonadonna P., Oude Elberink J.N.G., Golden D.B.K., Epidemiology, diagnosis, and treatment of Hymenoptera venom allergy in mastocytosis patients, Immunol. Allergy Clin. North Am., 2014; 34: 365-381
13. Bonadonna P., Bonifacio M., Lombardo C., Zanotti R., Hymenoptera allergy and mast cell activation syndromes, Curr. Allergy Asthma Rep., 2016; 16: 5
14. Bonadonna P., Gonzalez-de-Olano D., Zanotti R. y cols., Venom immunotherapy in patients with clonal mast cell disorders: efficacy, safety, and practical considerations, J. Allergy Clin. Immunol. Pract., 2013; 1: 474-478
15. Valent P., Akin C., Doctor, I think I am suffering from MCAS: differential diagnosis and separating facts from fiction, J. Allergy Clin. Immunol. Pract., 2019; 7: 1109-1114
16. González de Olano D., de la Hoz Caballer B., Núñez Lopez R. y cols., Prevalence of allergy and anaphylactic symptoms in 210 adult and pediatric patients with mastocytosis in Spain: a study of the Spanish network on mastocytosis (REMA), Clin. Exp. Allergy, 2007; 37: 1547-1555
17. Castells M., Diagnosis and management of anaphylaxis in precision medicine, J. Allergy Clin. Immunol., 2017; 140: 321-333
18. Alvarez-Twose I., González de Olano D., Sánchez-Muñoz L. y cols., Validation of the REMA score for predicting mast cell clonality and systemic mastocytosis in patients with systemic mast cell activation symptoms, Int. Arch. Allergy Immunol., 2012; 157: 275-280
19. Valent P., Escribano L., Broesby-Olsen S. y cols., Proposed diagnostic algorithm for patients with suspected mastocytosis: a proposal of the European Competence Network on Mastocytosis, Allergy, 2014; 69: 1267-1274
20. Brockow K., Jofer C., Behrendt H., Ring J., Anaphylaxis in patients with mastocytosis: a study on history, clinical features and risk factors in 120 patients, Allergy, 2008; 63: 226-232
21. Gulen T., Hagglund H., Dahlen B., Nilsson G., High prevalence of anaphylaxis in patients with systemic mastocytosis – a single-centre experience, Clin. Exp. Allergy, 2014; 44: 121-129
22. Hartmann K., Escribano L., Grattan C. y cols., Cutaneous manifestations in patients with mastocytosis: consensus report of the European Competence Network on Mastocytosis; the American Academy of Allergy, Asthma & Immunology; and the European Academy of Allergology and Clinical Immunology, J. Allergy Clin. Immunol., 2016; 137: 35-45
23. Lange M., Nedoszytko B., Górska A. y cols., Mastocytosis in children and adults: clinical disease heterogeneity, Arch. Med. Sci., 2012; 8: 533-541
24. Georgin-Lavialle S., Lhermitte L., Dubreuil P. y cols., Mast cell leukemia, Blood, 2013; 121: 1285-1295
25. Galen B.T., Rose M.G., Darier’s sign in mastocytosis, Blood, 2014; 123: 1127
26. Lange M., Niedoszytko M., Nedoszytko B. y cols., Diffuse cutaneous mastocytosis: analysis of 10 cases and a brief review of the literature, J. Eur. Acad. Dermatol. Venereol., 2012; 26: 1565-1571
27. Heide R., Zuidema E., Beishuizen A. y cols., Clinical aspects of diffuse cutaneous mastocytosis in children: two variants, Dermatology, 2009; 219: 309-315
28. Wolff K., Komar M., Petzelbauer P., Clinical and histopathological aspects of cutaneous mastocytosis, Leuk. Res., 2001; 25: 519-528
29. Vitte J., Human mast cell tryptase in biology and medicine, Mol. Immunol., 2015; 63: 18-24
30. Butterfield J.H., Weiler C.R., Prevention of mast cell activation disorder-associated clinical sequelae of excessive prostaglandin D2 production, Int. Arch. Allergy Immunol., 2008; 147: 338-343
31. Picard M., Giavina-Bianchi P., Mezzano V., Castells M., Expanding spectrum of mast cell activation disorders: monoclonal and idiopathic mast cell activation syndromes, Clin. Ther., 2013; 35: 548-562
32. Bodemer C., Hermine O., Palmerini F. y cols., Pediatric mastocytosis is a clonal disease associated with D816V and other activating c-KIT mutations, J. Invest. Dermatol., 2010; 130: 804-815
33. Rausz E., Szilagyi A., Nedoszytko B. y cols., Comparative analysis of IL6 and IL6 receptor gene polymorphisms in mastocytosis, Br. J. Haematol., 2013; 160: 216-219
34. Peavy R.D., Metcalfe D.D., Understanding the mechanisms of anaphylaxis, Curr. Opin. Allergy Clin. Immunol., 2008; 8: 310-315
35. Nedoszytko B., Niedoszytko M., Lange M. y cols., Interleukin-13 promoter gene polymorphism -1112C/T is associated with the systemic form of mastocytosis, Allergy, 2009; 64: 287-294
36. Daley T., Metcalfe D.D., Akin C., Association of the Q576R polymorphism in the interleukin-4 receptor alpha chain with indolent mastocytosis limited to the skin, Blood, 2001; 98: 880-882
37. Lange M., Glen J., Zablotna M. y cols., Interleukin-31 polymorphisms and serum IL-31 level in patients with mastocytosis: correlation with clinical presentation and pruritus, Acta Derm. Venereol., 2017; 97: 47-53
38. Górska A., Gruchała-Niedoszytko M., Niedoszytko M. y cols., The role of TRAF4 and B3GAT1 gene expression in the food hypersensitivity and insect venom allergy in mastocytosis, Arch. Immunol. Ther. Exp. (Warsz.), 2016; 64: 497-503
39. Niedoszytko M., Bruinenberg M., van Doormaal J.J. y cols., Gene expression analysis predicts insect venom anaphylaxis in indolent systemic mastocytosis, Allergy, 2011; 66: 648-657
40. Hodgkinson C.A., Moore K.J., Nakayama A. y cols., Mutations at the mouse microphthalmia locus are associated with defects in a gene encoding a novel basic-helix-loop-helix-zipper protein, Cell, 1993; 74: 395-404
41. Lee Y.-N., Brandal S., Noel P. y cols., KIT signaling regulates MITF expression through miRNAs in normal and malignant mast cell proliferation, Blood, 2011; 117: 3629-3640
42. Lee S.-H., Lee J.-H., Lee J.-H., Kim D.-K., Involvement of MITF-A, an alternative isoform of mi transcription factor, on the expression of tryptase gene in human mast cells, Exp. Mol. Med., 2010; 42: 366-375
43. Tefferi A., Levine R.L., Lim K.-H. y cols., Frequent TET2 mutations in systemic mastocytosis: clinical, KITD816V and FIP1L1-PDGFRA correlates, Leukemia, 2009; 23: 900-904
44. Traina F., Visconte V., Jankowska A.M. y cols., Single nucleotide polymorphism array lesions, TET2, DNMT3A, ASXL1 and CBL mutations are present in systemic mastocytosis, PLoS One, 2012; 7: e43 090
45. Schwaab J., Schnittger S., Sotlar K. y cols., Comprehensive mutational profiling in advanced systemic mastocytosis, Blood, 2013; 122: 2460-2466
46. Damaj G., Joris M., Chandesris O. y cols., ASXL1 but not TET2 mutations adversely impact overall survival of patients suffering systemic mastocytosis with associated clonal hematologic non-mast-cell diseases, PLoS One, 2014; 9: e85 362
47. Jawhar M., Schwaab J., Schnittger S. y cols., Additional mutations in SRSF2, ASXL1 and/or RUNX1 identify a high-risk group of patients with KIT D816V(+) advanced systemic mastocytosis, Leukemia, 2016; 30: 136-143
48. Akin C., Mast cell activation syndromes presenting as anaphylaxis, Immunol. Allergy Clin. North Am., 2015; 35: 277-285
49. Pardanani A., How I treat patients with indolent and smoldering mastocytosis (rare conditions but difficult to manage), Blood, 2013; 121: 3085-3094
50. Escribano L., Akin C., Castells M. y cols., Mastocytosis: current concepts in diagnosis and treatment, Ann. Hematol., 2002; 81: 677-690
51. Bonifazi F., Jutel M., Biló B.M. y cols., Prevention and treatment of hymenoptera venom allergy: guidelines for clinical practice, Allergy, 2005; 60: 1459-1470
52. Bilo M.B., Pravettoni V., Bignardi D. y cols., Hymenoptera venom allergy: management of children and adults in clinical practice, J. Investig. Allergol. Clin. Immunol., 2019; 29: 180-205
53. Bonadonna P., Perbellini O., Passalacqua G. y cols., Clonal mast cell disorders in patients with systemic reactions to Hymenoptera stings and increased serum tryptase levels, J. Allergy Clin. Immunol., 2009; 123: 680-686
54. Dubois A.E.J., Mastocytosis and Hymenoptera allergy, Curr. Opin. Allergy Clin. Immunol., 2004; 4: 291-295
55. Niedoszytko M., de Monchy J., van Doormaal J.J. y cols., Mastocytosis and insect venom allergy: diagnosis, safety and efficacy of venom immunotherapy, Allergy, 2009; 64: 1237-1245
56. Reimers A., Müller U., Fatal outcome of a Vespula sting in a patient with mastocytosis after specific immunotherapy with honey bee venom, Allergy Clin. Immunol. Int. J. WAO Org., 2005; 17: 69-70
 Español
Español
 English
English
 українська
українська