Baumgartner H, De Backer J, Babu-Narayan SV, et al; ESC Scientific Document Group. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur Heart J. 2021 Feb 11;42(6):563-645. doi: 10.1093/eurheartj/ehaa554. PMID: 32860028.
Stout KK, Daniels CJ, Aboulhosn JA, et al. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019 Apr 2;139(14):e637-e697. doi:10.1161/CIR.0000000000000602. PMID: 30586768.
Definition, Etiology, PathogenesisTop
Atrial septal defects (ASDs) are the most common congenital heart disease diagnosed in adults, accounting for >30% of all defects seen in this population.
Types of ASD:
1) Ostium secundum ASD (70%-80%).
2) Ostium primum ASD (partial atrioventricular septal defect; 15%).
3) Sinus venosus ASD (superior sinus venosus defects [5%], inferior sinus venosus defects [<1%]).
4) Coronary sinus ASD (unroofed coronary sinus; <1%).
A common feature of all types of ASD is an atrial level shunt and its consequences, while differences include location of the defect and other coexisting heart defects. Approximately 30% of all ASDs are associated with other cardiac malformations, most common of which are anomalous pulmonary venous drainage (seen in all patients with superior sinus venosus defects), ventricular septal defects, and patent ductus arteriosus. All patients with ostium primum defects have abnormality of the mitral valve (a cleft anterior leaflet) with a varying degree of regurgitation.
Clinical Features and Natural HistoryTop
1. Symptoms: The most common symptom is dyspnea on exertion with progressive reduction of exercise tolerance and palpitations, which are initially paroxysmal (usually caused by atrial fibrillation [AF] or atrial flutter). For many women with undiagnosed defects the initial symptoms are noticed during pregnancy. Rarely initial symptoms may be related to paradoxical embolization.
2. Signs: Typically physical examination findings are quite subtle and can be missed. The jugular venous pulse may appear normal. However, a wave dominance may be noticed, and in the presence of severe tricuspid regurgitation (TR) a cv wave will be clearly visible. A right ventricular lift is present in patients with significant right ventricular enlargement. A classic finding of an ASD on auscultation is a fixed splitting of the second heart sound. Special attention should be paid to the intensity of the pulmonic component. A loud pulmonic valve component of the second heart sound would indicate the presence of significant pulmonary hypertension. Many patients with an ASD will also have a systolic outflow murmur audible over the main pulmonary artery, which is related to increased flow across the right ventricular outflow tract. In individuals with very large shunts, a soft mid-diastolic rumble in the fourth left intercostal space may be heard, indicative of a significant increase in flow across the tricuspid valve. Patients may also have a holosystolic murmur caused by secondary TR. In the case of significant TR, its symptoms may be dominant, sometimes including central cyanosis (when the regurgitant jet is directed through the ASD to the left atrium).
3. Natural history: There is a direct link between the size of the defect and associated morbidity. The presence of additional cardiac defects will influence treatment and mortality. Patients with ostium secundum ASD have a relatively benign course and can remain asymptomatic for decades. Symptoms usually appear in the third to fourth decades of life and are generally progressive. The overall survival of patients is close to that of the healthy population, but the quality of life is impaired due to progressive heart failure, sustained AF or atrial flutter, and dyspnea. Severe pulmonary hypertension is very rare (<5%).
DiagnosisTop
The final diagnosis is based on echocardiography, less commonly on magnetic resonance imaging (MRI) or computed tomography (CT). Suspected increased pulmonary vascular resistance is an indication for cardiac catheterization prior to referral for surgical or interventional closure.
1. Electrocardiography (ECG):
1) Ostium secundum ASD (Figure 3.7-1): Incomplete right bundle branch block (RBBB), in 90% of cases with rsR’ pattern, right axis deviation or rightward axis, usually normal but possibly tall or wide P waves, features of right ventricular overload (prominent R in V1 and V2), and supraventricular arrhythmias (frequently AF and atrial flutter).
2) Ostium primum ASD (Figure 3.7-2): First-degree atrioventricular block in some patients, incomplete RBBB, features of right ventricular overload (prominent R in V1 and V2) and left axis deviation. Evidence of left atrium enlargement in those with significant mitral regurgitation.
3) Sinus venosus defect: Incomplete RBBB, right axis deviation or rightward axis, abnormal P-wave axis with negative P waves in leads II, III, and aVF.
2. Chest radiography (Figure 3.7-3): Increased pulmonary perfusion (shunt vascularity), dilated right ventricle and main pulmonary artery, narrow aorta.
3. Echocardiography (Figure 3.7-4, Figure 3.7-5): Transthoracic echocardiography (TTE) allows visualization of the defect and cardiac remodeling secondary to the shunt as well as visualization of the shunt using color Doppler imaging. In some individuals calculation of the pulmonary (Qp) to systemic (Qs) flow ratio is possible with good accuracy (shunt is significant in patients with Qp:Qs ≥1.5). Calculation of the right ventricular systolic pressure, which in those without right ventricular outflow tract obstruction equals the pulmonary artery systolic pressure, is an important component of echocardiographic evaluation. Additionally, detection of associated heart defects is possible. In equivocal cases transesophageal echocardiography (TEE) is conclusive; this is necessary when transcatheter closure of the defect is considered (in ostium secundum ASDs only). Sinus venosus defects are very difficult to visualize on TTE and are routinely missed. Hence, unexplained right heart enlargement on TTE should raise the suspicion of a sinus venosus defect, anomalous pulmonary venous drainage, or both and requires TEE examination.
4. Cardiac catheterization: Although there is no role for routine cardiac catheterization in patients with ASDs, it is performed in patients being considered for ASD closure with:
1) Established coronary artery disease (CAD).
2) Age >40 years.
3) A significant risk factor profile for CAD.
4) Significant pulmonary hypertension (to assess pulmonary vascular reactivity).
5. Cardiac MRI: Cardiac MRI provides a robust noninvasive way of assessing not only cardiac anatomical anomalies such as ASDs and anomalous pulmonary venous connections, but it can also provide accurate measures of the shunt ratio (Qp:Qs) as well as ventricular volumes and function. In many centers this modality has become a routine part of assessment of patients with shunt lesions.
TreatmentTop
1. Patients with minor shunts, normal right heart size, and normal pulmonary pressures require neither treatment nor specific recommendations. However, there is a small risk of paradoxical embolization; such risk is especially important in patients requiring intracardiac device therapy, such as permanent pacemakers or implantable defibrillators. This is due to the development of microthrombi on intracardiac leads, which can then embolize paradoxically to the central nervous system. In these patients percutaneous closure of the defect (ostium secundum ASDs only) should be considered prior to device implantation.
2. Patients with significant left-to-right shunts (with right ventricular volume overload) require invasive treatment; pharmacotherapy with diuretics can only provide symptomatic relief. Invasive treatment should also be considered in the case of paradoxical embolism (after other causes have been excluded). Closure of the defect is contraindicated in patients with severe pulmonary hypertension (pulmonary artery systolic pressure >2/3 systolic blood pressure or pulmonary hypertension and pulmonary vascular resistance ≥5 Wood units) or Eisenmenger syndrome. Transcatheter (percutaneous) closure is only suitable for ostium secundum ASDs and may be used in eligible patients (depending on the size of the defect/defects); following the procedure, both dual antiplatelet therapy (acetylsalicylic acid [ASA] 75-100 mg/d and clopidogrel 75 mg/d or ticlopidine 250 mg bid; treatment regimens may be slightly different in various centers) and infective endocarditis prophylaxis should be administered for a total of 6 months. After a successful closure of the defect, many patients have a significant reduction in the right heart size. Requirements for ongoing follow-up depend on the size of the defect and associated abnormalities, presence and degree of pulmonary hypertension (more than mild) prior to closure, and age of the patient at closure (closed in adulthood).
3. Severe TR: In patients with ostium secundum ASDs and severe TR, surgical treatment of the ASD with concomitant repair of the tricuspid valve is necessary.
4. Ostium primum ASDs: All patients with ostium primum ASDs have associated cardiac defects, which include a cleft anterior mitral valve leaflet leading to a variable degree of mitral regurgitation and in many patients inlet ventricular septum defects. All patients with hemodynamically significant shunts will require surgical intervention with closure of the defect/defects and repair of the mitral valve.
5. Sinus venosus defects: All patients with superior sinus venosus defects have associated anomalous pulmonary venous drainage and/or connection and require surgical intervention for closure of the defect and rerouting the anomalous vein to the left atrium.
Complications and PrognosisTop
The prognosis is directly related to the size of the defect, degree of pulmonary hypertension (prior to closure), and age at closure. Some of the complications of untreated ASDs are supraventricular arrhythmias, rarely sick sinus syndrome or complete heart block (sometimes as a consequence of surgery), Eisenmenger syndrome, and paradoxical embolism (during intraventricular pacing). Patients without significant pulmonary hypertension and Eisenmenger syndrome have a favorable prognosis.
FiguresTop
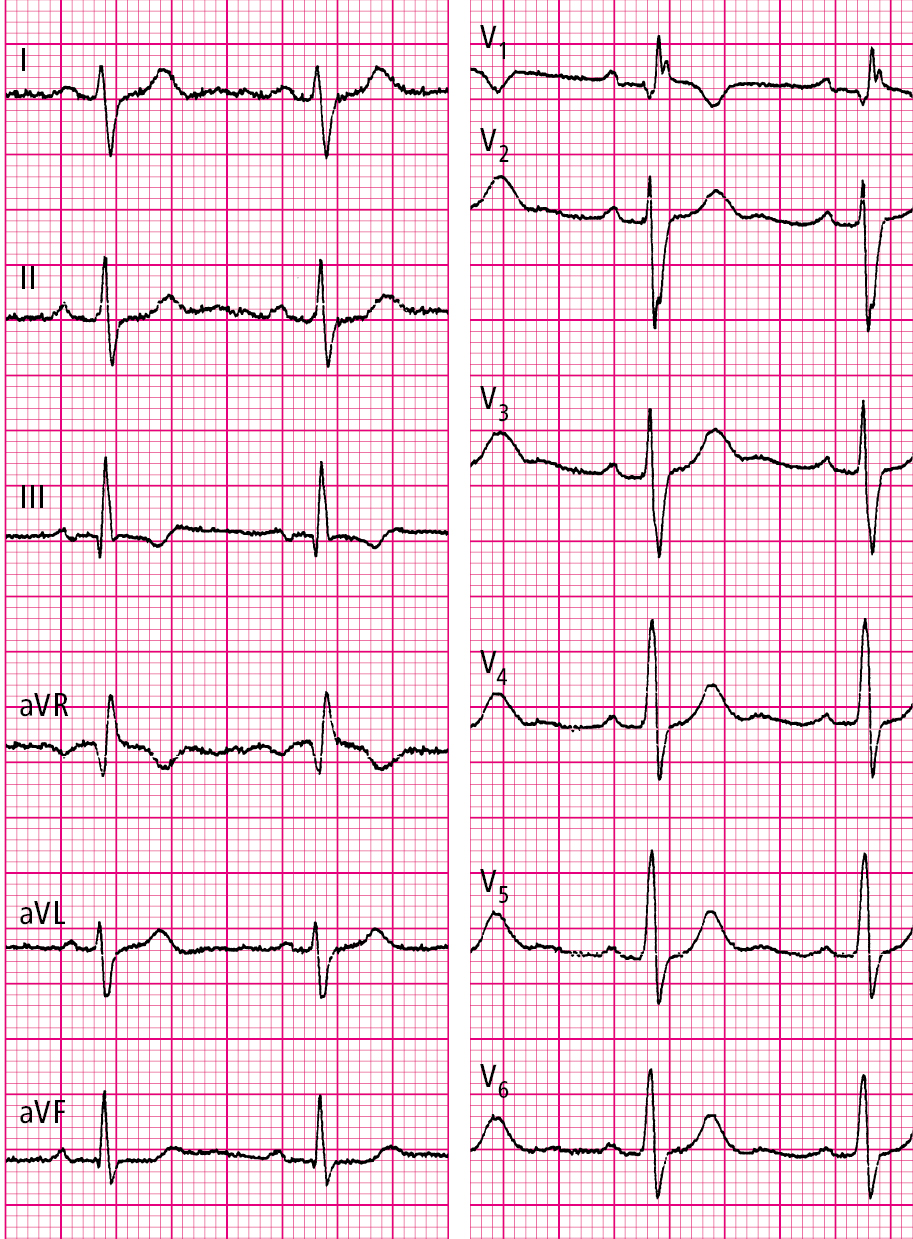
Figure 3.7-1. Electrocardiography (ECG) of a patient with secundum atrial septal defect (ASD): normal sinus rhythm, 77 beats/min, right axis deviation, incomplete right bundle branch block (RBBB), features of right ventricular hypertrophy.
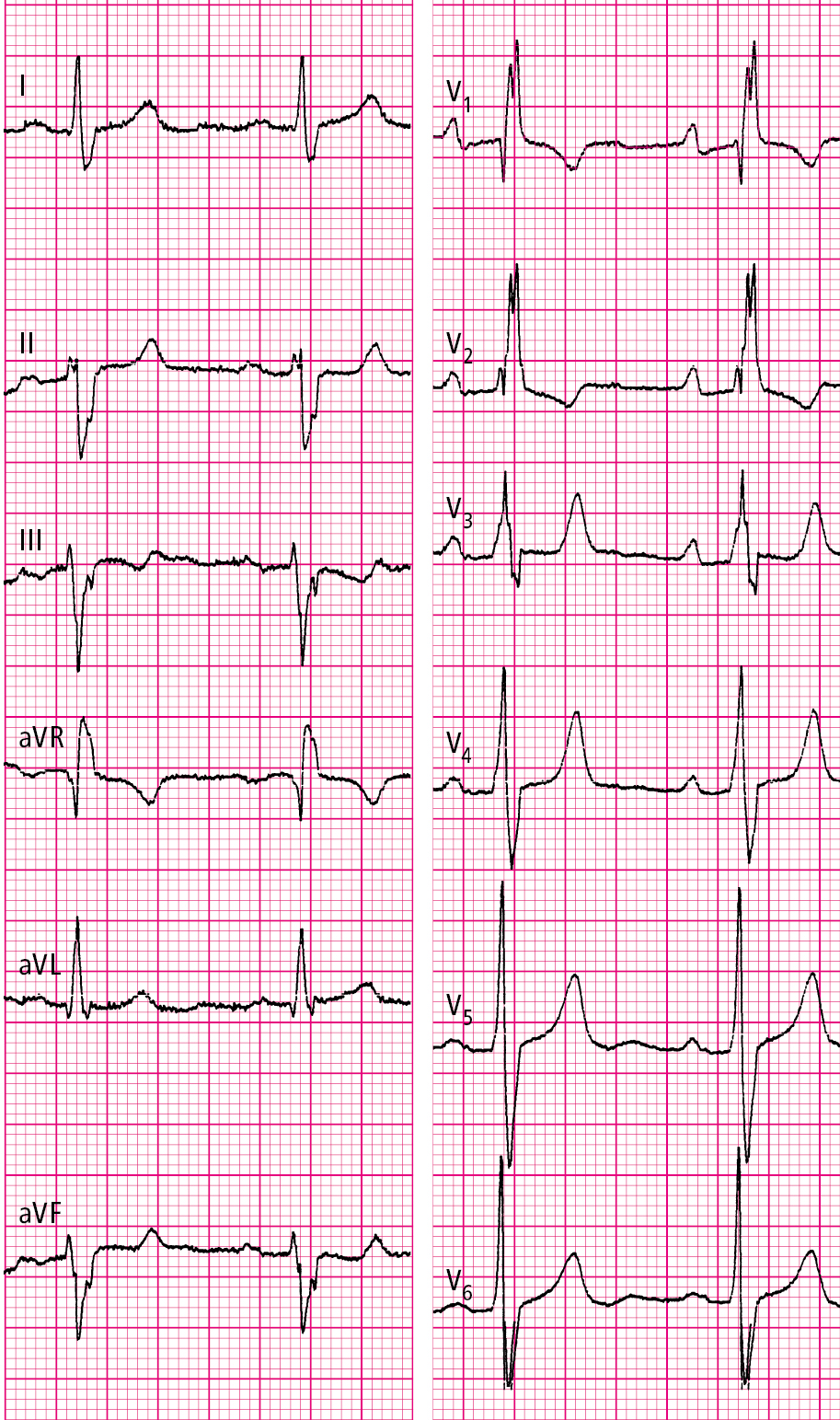
Figure 3.7-2. Electrocardiography (ECG) of a patient with primum atrial septal defect (ASD): normal sinus rhythm, 69 beats/min, left axis deviation, right bundle branch block (RBBB), features of right ventricular hypertrophy.
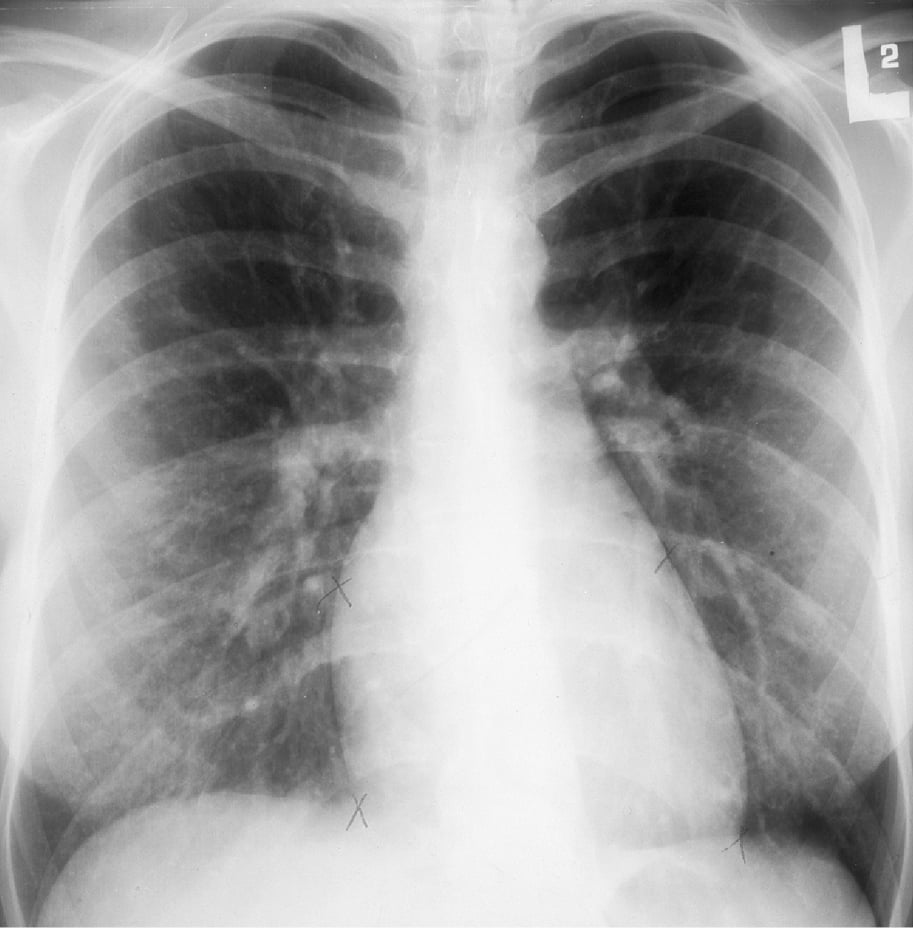
Figure 3.7-3. Chest radiography of a patient with secundum atrial septal defect (ASD): dilated pulmonary arteries, features of increased pulmonary blood flow.
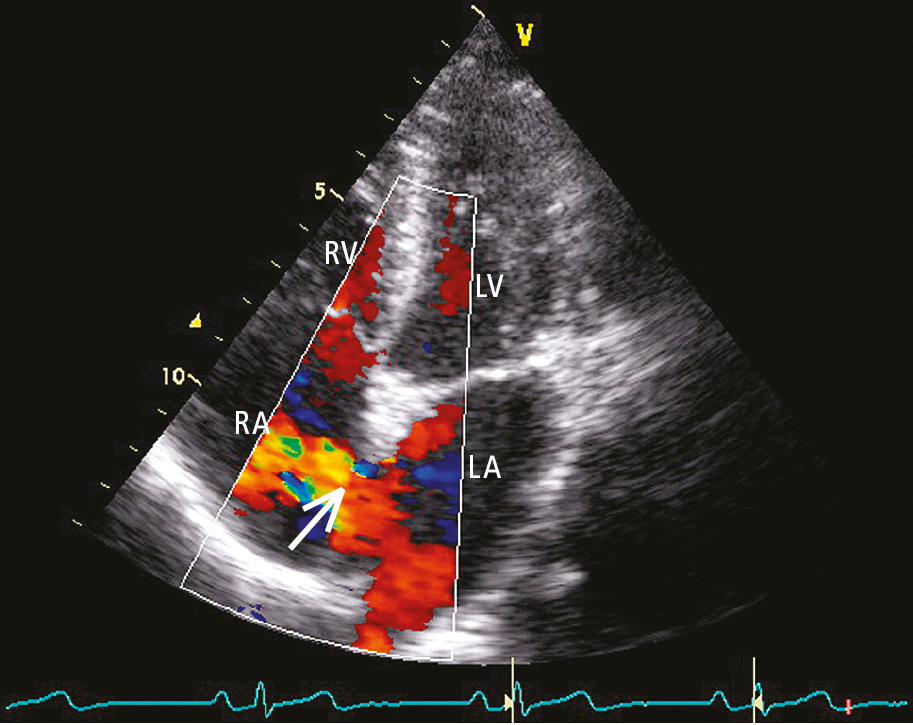
Figure 3.7-4. Transthoracic echocardiography (TTE) (4-chamber view): secundum atrial septal defect (ASD). Color Doppler imaging shows a left-to-right shunt (arrow). LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
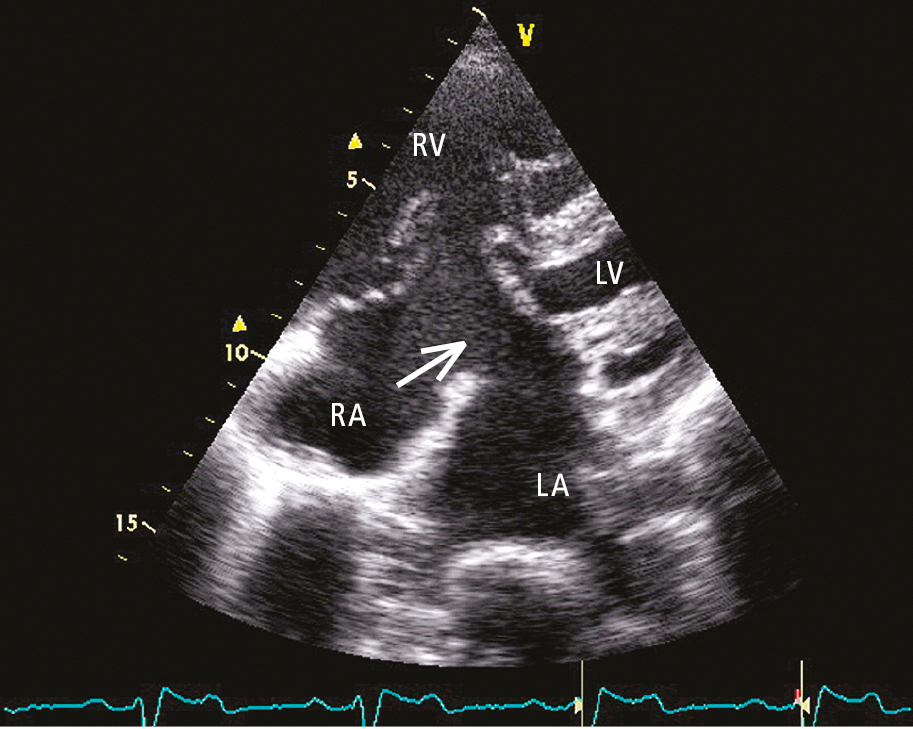
Figure 3.7-5. Transthoracic echocardiography (TTE) (4-chamber view): primum atrial septal defect (ASD). Discontinuity of the interatrial septum (arrow). LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.