PathophysiologyTop
Acetaminophen (INN paracetamol) poisoning is usually a result of an intentional (suicidal) overdose of oral formulations but may be also accidental from high doses taken for analgesia or due to accumulation of the drug in patients with severe liver disease. Ten-fold errors in the administration of parenteral acetaminophen have also resulted in toxicity.
In acute acetaminophen poisoning the liver is the critical organ with hepatocyte injury and necrosis. Acute kidney injury due to proximal renal tubule damage is less frequent, affecting 25% of patients with severe liver damage and 50% of patients with liver failure caused by acetaminophen poisoning.
Peak serum acetaminophen levels after oral ingestion of tablets or capsules occur after 2 to 4 hours. Hepatotoxicity can occur in adults who take a single dose of 6 to 7 g and toxicity may occur with ingestion of 150 mg/kg/d for ≥2 days.
Clinical Features and DiagnosisTop
Phase 1 (up to 24 hours from ingestion) is asymptomatic in most patients. Some individuals may have nausea and vomiting or, less frequently, abdominal pain, excessive sweating, pallor, and weakness. In very rare cases severe manifestations (coma, severe lactate acidosis) develop as early as on the first day from ingestion; this is characteristic for severe overdoses (75-100 g).
Phase 2 (24-72 hours): Pain or tenderness in the right upper abdominal quadrant, jaundice. Symptoms are accompanied by an increase in serum aminotransferase (alanine aminotransferase [ALT], aspartate aminotransferase [AST]) and bilirubin levels, international normalized ratio (INR), as well as hypoglycemia and metabolic acidosis.
Phase 3 (72-96 hours): Fulminant liver failure with encephalopathy and less frequently bleeding. An increase in serum creatinine levels is usually observed on days 2 to 5 from ingestion.
Phase 4 (death or organ regeneration): Death due to fulminant liver failure usually occurs on days 3 to 5 from ingestion. In surviving patients normalization of laboratory parameters and organ regeneration occur in 7 to 14 days from ingestion. Generally recovery of hepatic function is quite remarkable in survivors that do not need transplant.
Serum acetaminophen levels should be measured not earlier than 4 hours from ingestion. If the level is drawn within 4 hours, it should be repeated at the 4-hour mark, unless it was nondetectable. If the level at 4 hours is borderline or there is suspected delayed absorption, measurement should be repeated at 8 hours. It is crucial to measure acetaminophen levels at 4 to 8 hours from ingestion and administer the antidote (N–acetylcysteine [NAC]), as its efficacy is highest in this period.
High anion gap metabolic acidosis can sometimes be associated with chronic acetaminophen toxicity, independent of hepatic damage, due to 5-oxoproline (pyroglutamic acid) accumulation. This is different from the lactic acidosis that may develop as a result of hepatic injury in acute overdose. Risk factors include chronic acetaminophen use (usually patients have serum values of acetaminophen well below the toxic range), female sex, malnutrition, chronic kidney disease, and pregnancy.
Other studies: Serum aminotransferase, urea/blood urea nitrogen (BUN), creatinine, bilirubin, lactate, and phosphate levels; INR; arterial blood gas analysis.
The Ontario Poison Center has recently changed its recommended management and testing protocol for acetaminophen poisoning. The current recommendations are as follows:
1) On initial presentation of all patients with suspected acetaminophen overdose:
a) Acetaminophen level (≥4 hours after the end of ingestion).
b) Acetylsalicylic acid (ASA) level.
c) Venous or arterial blood gases, electrolytes (Na, K, Cl, HCO3), glucose, BUN, creatinine, osmolality.
d) AST, ALT, INR.
e) Ethanol level, depending on clinical scenario.
2) Sustained-release preparations or when opioid or anticholinergic drugs were ingested concurrently: Repeat the acetaminophen level every 4 hours until it peaks, then repeat measurements every 12 hours until the level is undetectable (<66 micromol/L [10 microg/dL]).
3) Patients receiving NAC:
a) Repeat venous gases, electrolytes, glucose, BUN, creatinine, AST, ALT, and INR every 12 hours.
b) Repeat the acetaminophen level every 12 hours until it is undetectable.
4) Patients determined to be at severe risk:
a) Lactate and lipase levels.
b) Phosphate (PO4) levels if liver enzymes are elevated.
c) Repeat acetaminophen level, venous gases, electrolytes, glucose, BUN, creatinine, AST, ALT, and INR every 4 hours until the acetaminophen level peaks and then every 12 hours until it is undetectable.
TreatmentTop
1. Decontamination: Administration of activated charcoal (1 g/kg in adults) within 1 to 2 hours. Activated charcoal can be administered after 2 hours if extended-release formulations of acetaminophen were ingested and repeat doses can be considered for massive ingestions. Gastric lavage may be used for massive ingestions in patients presenting within an hour of ingestion.
2. Antidote: NAC. IV administration is preferred because the duration of treatment is shorter compared with oral administration. Administration of NAC is associated with risk of a nonallergic anaphylactoid response (urticaria, angioedema, bronchospasm, or hypotension that typically resolve with stopping or slowing the infusion and providing supportive care). However, in patients with absolute indications for the treatment NAC is administered despite the adverse effects. The decision on the administration of NAC within 24 hours of poisoning is made based on a nomogram of acetaminophen blood levels (Figure 19.2-1). The administration of NAC should be started when the measured drug level falls on or is above the curve. In patients admitted >24 hours from poisoning or in whom the time of poisoning cannot be established, start NAC administration immediately.
Based on recent literature suggesting safer administration and easier dosing strategies, the Ontario and Manitoba Poison Control Centre has changed its suggested dosing for NAC. The solution concentration has been standardized to minimize preparation errors and the loading dose is the same for both high-risk and low-risk (typical) patients.
A standard NAC solution of 30 mg/mL is prepared for all patients. At our site, this is prepared by adding 15,000 mg of NAC to achieve a total volume of 500 mL in a bag of 5% glucose (dextrose). The loading dose is 60 mg/kg/h for 4 hours (a maximum dose based on 100 kg). Maintenance dosing is continued for ≥12 hours or until the criteria for stopping are met.
In a typical scenario after the loading dose infusion is continued at a rate of 6 mg/kg/h. In a high-risk scenario (patients with massive ingestion, evidence of liver dysfunction at presentation, or those needing hemodialysis) after the loading dose infusion is continued at 12 mg/kg/h.
Stopping criteria:
1) Acetaminophen level is nondetectable (<66 micromol/L [10 microg/dL]); and
2) AST/ALT levels are <100 IU/L (or decreasing and <50% of peak levels); and
3) INR is <2.0; and
4) The patient is well; and
5) A minimum of 12 hours (including the loading dose) of NAC has been given.
3. Methods of enhanced elimination: Hemodialysis. In patients with severe acetaminophen overdose (serum drug levels >5300 micromol/L [5.3 mmol/L]) associated with coma and metabolic acidosis, consider urgent hemodialysis; use a higher rate of NAC infusion (see above) for patients requiring dialysis. There are no established criteria for starting hemodialysis in patients with acetaminophen toxicity and it is not routinely used. Therefore, the decision to proceed with this intervention should be closely discussed with toxicology nephrology services.
4. Liver transplant: Patients are qualified for transplant using the King’s College criteria (see Acute Liver Failure).
FiguresTop
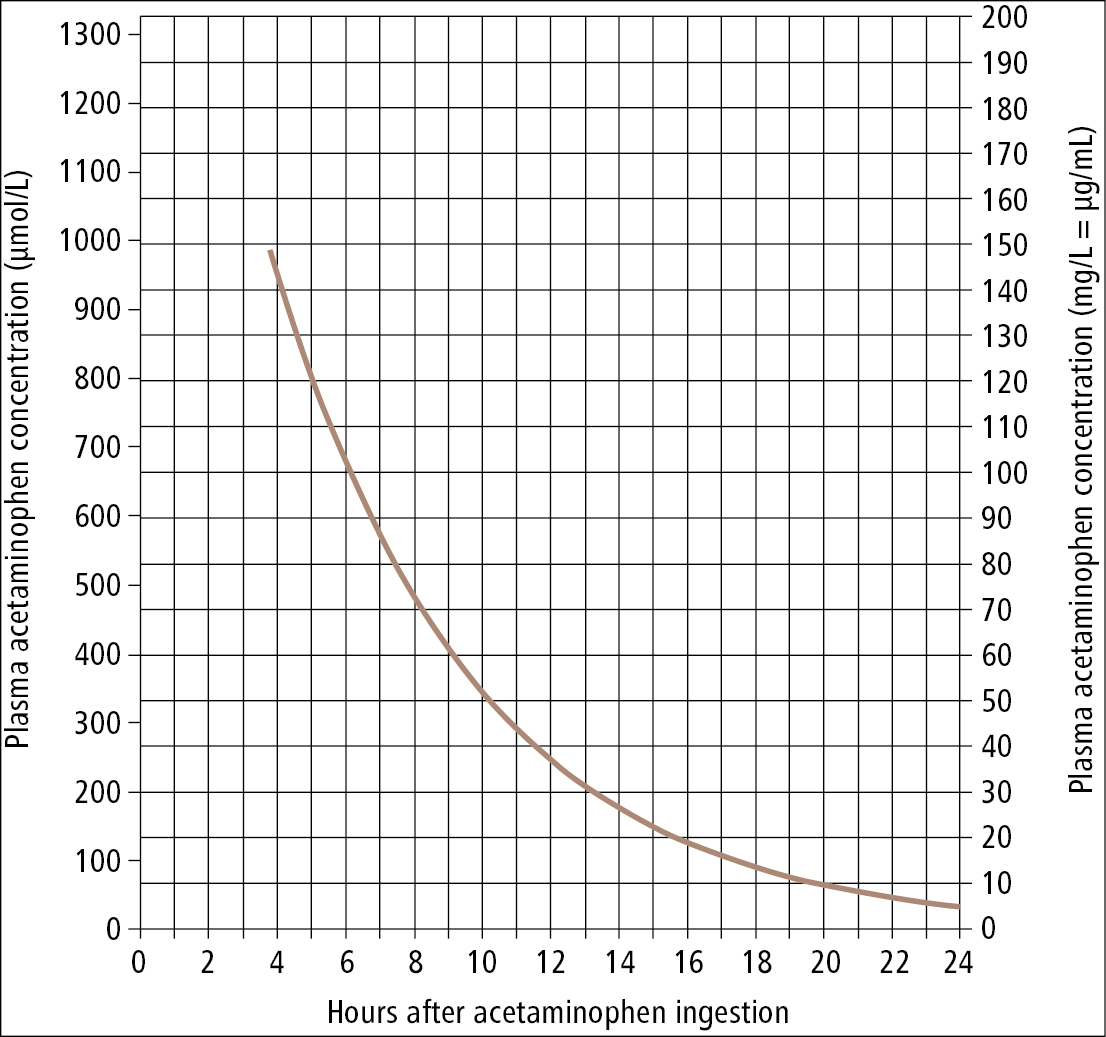
Figure 19.2-1. A decision-making nomogram for the use of antidotes in the treatment of acetaminophen overdose based on serum acetaminophen levels and time from ingestion. Adapted from protocols by the Ontario Poison Centre.