Averbuch D, Orasch C, Cordonnier C, et al; ECIL4, a joint venture of EBMT, EORTC, ICHS, ESGICH/ESCMID and ELN. European guidelines for empirical antibacterial therapy for febrile neutropenic patients in the era of growing resistance: summary of the 2011 4th European Conference on Infections in Leukemia. Haematologica. 2013 Dec;98(12):1826-35. doi: 10.3324/haematol.2013.091025. Erratum in: Haematologica. 2014 Feb;99(2):400. PubMed PMID: 24323983; PubMed Central PMCID: PMC3856957.
Freifeld AG, Bow EJ, Sepkowitz KA, et al; Infectious Diseases Society of America. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of America. Clin Infect Dis. 2011 Feb 15;52(4):e56-93. doi: 10.1093/cid/cir073. PubMed PMID: 21258094.
Aapro MS, Bohlius J, Cameron DA, et al; European Organisation for Research and Treatment of Cancer. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011 Jan;47(1):8-32. doi: 10.1016/j.ejca.2010.10.013. PubMed PMID: 21095116.
Definition, Etiology, PathogenesisTop
Neutropenia is the most common hematologic complication of cancer treatment. It may result from the myelotoxic effects of chemotherapy or radiation therapy or from bone marrow infiltration by malignant cells. In patients with febrile neutropenia the causative pathogen can be identified only in 20% to 30% of cases. Prior to the introduction of empiric therapy for febrile neutropenia, the most frequently identified etiologic agents were Pseudomonas spp and Enterobacteriaceae (eg, Escherichia coli, Klebsiella spp) followed by gram-positive cocci (most commonly Staphylococcus aureus). In the era of empiric therapy, there has been a shift in the microbiology of pathogens to predominantly gram-positive organisms (coagulase-negative staphylococci being the most common) followed by Enterobacteriaceae and then by nonfermenting gram-negative bacilli, such as Pseudomonas aeruginosa.
Febrile neutropenia is defined as:
1) Oral temperature ≥38.3 degrees Celsius in a single measurement or ≥38 degrees Celsius sustained over ≥1 hour; and
2) Absolute neutrophil count (ANC) <0.5×109/L or an expected decrease in ANC <0.5×109/L within 48 hours.
ManagementTop
1. Assess the risk of complications and death using the Multinational Association of Supportive Care in Cancer (MASCC) score (Table 10.5-1) or a simplified classification:
1) High risk: Expected long-term (>7 days) and profound neutropenia (ANC ≤0.1×109/L) and/or clinically significant complications.
2) Low risk: All other patients.
2. Collect ≥2 separate specimens for blood cultures, every time from each lumen of a central vascular catheter, peripheral vein, and from other sites, depending on the suspected etiology (see Peripheral Venous Blood Sampling).
3. Immediately start empiric febrile neutropenia therapy (Figure 10.5-1) using an escalation strategy. This strategy, avoiding initial combination therapies and carbapenems, could be used in patients with no previous infections or colonization with resistant bacteria and in centers where infections due to resistant pathogens are rarely seen.
4. With the escalation strategy being the standard of care, in some cases a de-escalation strategy may be employed. Examples include initial therapy involving carbapenems as the first-line regimens (in seriously ill patients, eg, with septic shock; known previous colonization with extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae or gram-negative bacteria resistant to narrow-spectrum beta-lactam antibiotics or centers with high prevalence of such strains) or combinations with aminoglycosides (septic shock, resistant Pseudomonas or Acinetobacter strains likely due to colonization or local epidemiology, or use of carbapenems within the previous month). Subsequent management is directed to treat the identified infection (even if it is culture-negative) and to narrow the coverage (Figure 10.5-2).
Indications for adding vancomycin or another antibiotic active against gram-positive bacteria to the initial empiric therapy:
1) Hemodynamic instability or other signs of severe sepsis.
2) Radiographically confirmed pneumonia.
3) Cultures positive for gram-positive bacteria (prior to the definitive identification of the causative pathogen and obtaining drug susceptibility test results).
4) Clinical suspicion of a severe vascular catheter-associated infection (eg, chills during an infusion via the catheter or signs of infection in the area adjacent to the catheter).
5) Skin or soft tissue infection.
6) Documented colonization by methicillin-resistant S aureus (MRSA) or penicillin-resistant pneumococci.
7) Severe mucositis in patients who received prophylactic fluoroquinolones and their current empiric therapy includes ceftazidime.
Modification of the empiric febrile neutropenia therapy in the case of suspected or confirmed infection with a resistant strain (according to 2011 Infectious Diseases Society of America and 2013 European Conference on Infections in Leukemia guidelines):
1) MRSA: Vancomycin, linezolid, or daptomycin.
2) Vancomycin-intermediate S aureus (VISA): Linezolid, tigecycline, daptomycin, quinupristin/dalfopristin.
3) Vancomycin-resistant enterococci (VRE): Linezolid, tigecycline, daptomycin, quinupristin/dalfopristin (Enterococcus faecium).
4) ESBL-producing strains: Carbapenem.
5) Carbapenem-resistant Enterobacteriaceae strains: Colistin, tigecycline, or an aminoglycoside.
6) Carbapenemase-producing Klebsiella pneumoniae: Colistin or tigecycline.
7) Beta-lactamase–resistant P aeruginosa: Colistin or aminoglycosides.
8) Beta-lactamase–resistant Acinetobacter spp: Colistin or tigecycline.
9) Stenotrophomonas maltophilia: Trimethoprim/sulfamethoxazole, fluoroquinolones (ciprofloxacin, moxifloxacin), ticarcillin/clavulanate.
Patients in whom the etiologic agent responsible for the infection has been identified should receive a targeted therapy. However, it is recommended that empiric febrile neutropenia coverage be maintained until the resolution of fever and neutrophil recovery (ANC >0.5×109/L).
Between days 2 through 4 of empiric antibiotic therapy reevaluate the patient: Figure 10.5-3. Patients with persistent neutropenia who have completed a course of antibiotics should stay in the hospital for an observation lasting ≥24 to 48 hours. Management of high-risk patients with fever persisting for >4 days of empiric antibiotic therapy should include either the addition of empiric antifungal therapy or preemptive therapy (therapy as directed by testing indicating the possibility of fungal infection [bronchoalveolar lavage, galactomannan, high-resolution computed tomography]) in selected patients: Figure 10.5-4.Evidence 1Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). Low Quality of Evidence (low confidence that we know true effects of the intervention). Quality of Evidence lowered due to the risk of bias and a small number of events. Note: Although this is considered standard of care, it is based on low quality of evidence with issues in the methodology, small sample size, and potential for bias in the assessment of fungal infection. There was a reduction in invasive fungal infections (relative risk, 0.25; 95% CI, 0.12-0.54; 5 randomized controlled trials with n=800) but no effect on all-cause mortality. Sensitivity analysis according to study quality and time of treatment commencement did not alter the finding; the number needed to treat to prevent one invasive fungal infection was 17, and control rate was 7.7%. Goldberg E, Gafter-Gvili A, Robenshtok E, Leibovici L, Paul M. Empiric antifungal therapy for patients with neutropenia and persistent fever: Systematic review and meta-analysis. Eur J Cancer. 2008 Oct;44(15):2192-203. doi: 10.1016/j.ejca.2008.06.040. Review. PubMed PMID: 18706808.
5. Prophylaxis in afebrile neutropenic patients:
1) Follow universal precautions, particularly hand hygiene and cough etiquette (masks covering the face and nose), for all patients. Institute reverse isolation with laminar flow or high-efficiency particulate air (HEPA) filtration only for hematopoietic stem cell transplant patients and those receiving standard therapy for acute leukemia or aplastic anemia.
2) Recommend a fluoroquinolone (ciprofloxacin or levofloxacin) or trimethoprim/sulfamethoxazole only in high-risk patients, defined as hematologic patients on standard chemotherapy, hematopoietic stem cell transplant patients, and patients with solid tumors (breast, lung, ovary, germ cell tumors).Evidence 2Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of the intervention). Gafter-Gvili A, Fraser A, Paul M, et al. Antibiotic prophylaxis for bacterial infections in afebrile neutropenic patients following chemotherapy. Cochrane Database Syst Rev. 2012 Jan 18;1:CD004386. doi: 10.1002/14651858.CD004386.pub3. Review. PubMed PMID: 22258955; PubMed Central PMCID: PMC4170789.
3) Recommend antifungal and antiviral agents only in patients undergoing allogeneic hematopoietic stem cell transplant or induction or reinduction chemotherapy for acute myeloid leukemia.Evidence 3Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of the intervention). Bow EJ, Laverdière M, Lussier N, Rotstein C, Cheang MS, Ioannou S. Antifungal prophylaxis for severely neutropenic chemotherapy recipients: a meta analysis of randomized-controlled clinical trials. Cancer. 2002 Jun 15;94(12):3230-46. PubMed PMID: 12115356. Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007 Jan 25;356(4):348-59. PubMed PMID: 17251531. Gøtzsche PC, Johansen HK. Routine versus selective antifungal administration for control of fungal infections in patients with cancer. Cochrane Database Syst Rev. 2014 Sep 4;(9):CD000026. doi: 10.1002/14651858.CD000026.pub2. Review. PubMed PMID: 25188768.
4) Recommend trimethoprim/sulfamethoxazole in patients with risk factors for Pneumocystis jiroveci infection, for instance, in the course of glucocorticoid therapy lasting ≥1 month or therapy with purine analogues and allogeneic transplant recipients after engraftment.
5) Consider granulocyte colony-stimulating factor or granulocyte and macrophage colony-stimulating factor in patients with prolonged bacteremia and confirmed infections.
6) Avoid prolonged contacts with environment contaminated with fungal spores (eg, large-scale construction/renovation works).
Tables and FiguresTop
|
Characteristic |
Score |
|
Burden of illness (select only one): – No or mild symptoms – Moderate symptoms – Severe illness or terminal condition |
5 3 0 |
|
No hypotension |
5 |
|
No COPD |
4 |
|
Solid tumor or hematologic malignancy with no previous fungal infection |
4 |
|
No dehydration requiring parenteral fluids |
3 |
|
Outpatient at the onset of fever |
3 |
|
Age <60 years (not applicable to age ≤16 years) |
2 |
|
Interpretation: Score ≥21: Low risk | |
|
Source: J Clin Oncol. 2000;18(16):3038-51. | |
|
COPD, chronic obstructive pulmonary disease; MASCC, Multinational Association of Supportive Care in Cancer. | |
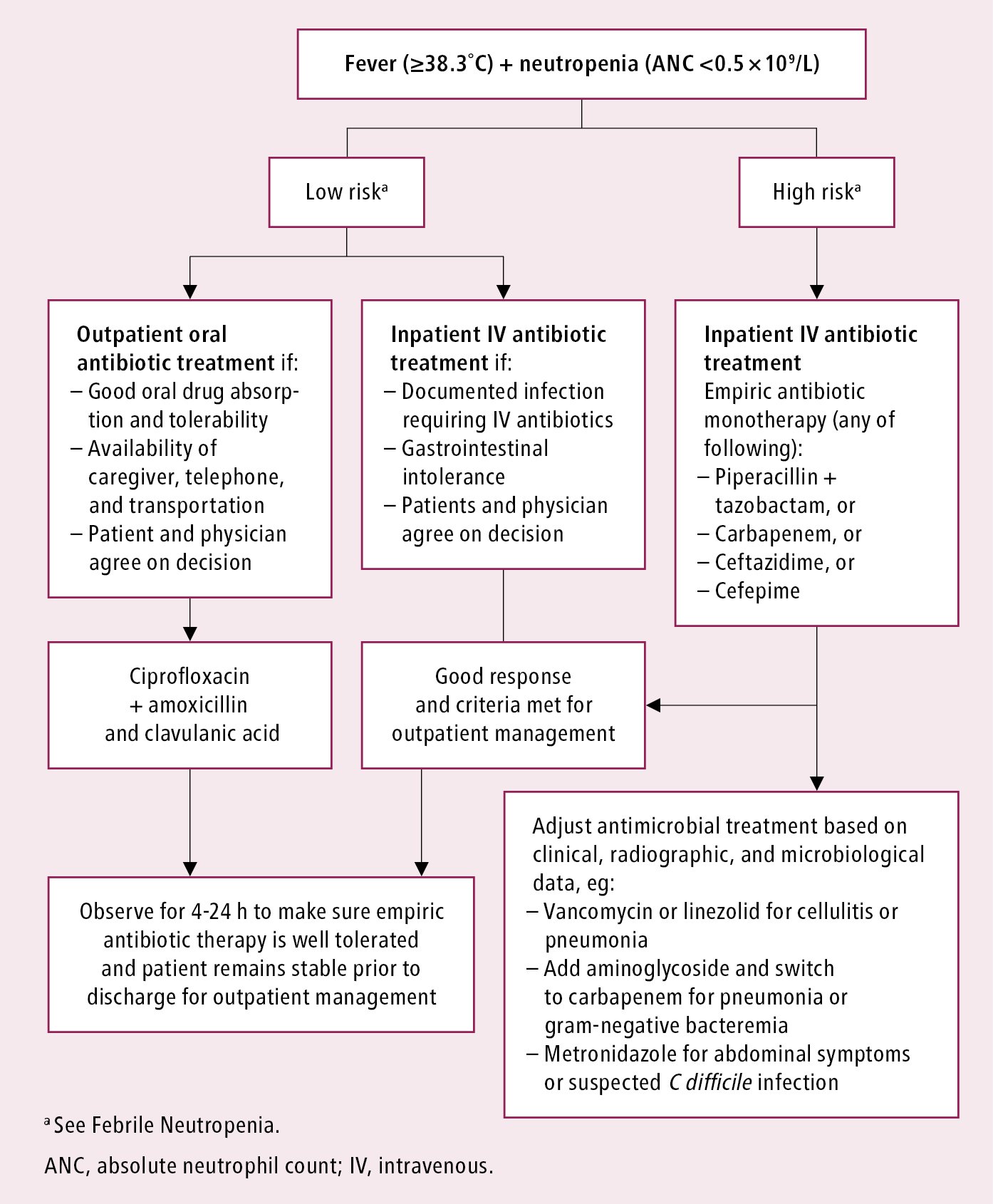
Figure 10.5-1. Initial management of patients with fever and neutropenia. Source: Clin Infect Dis. 2011;52(4):e56-93.
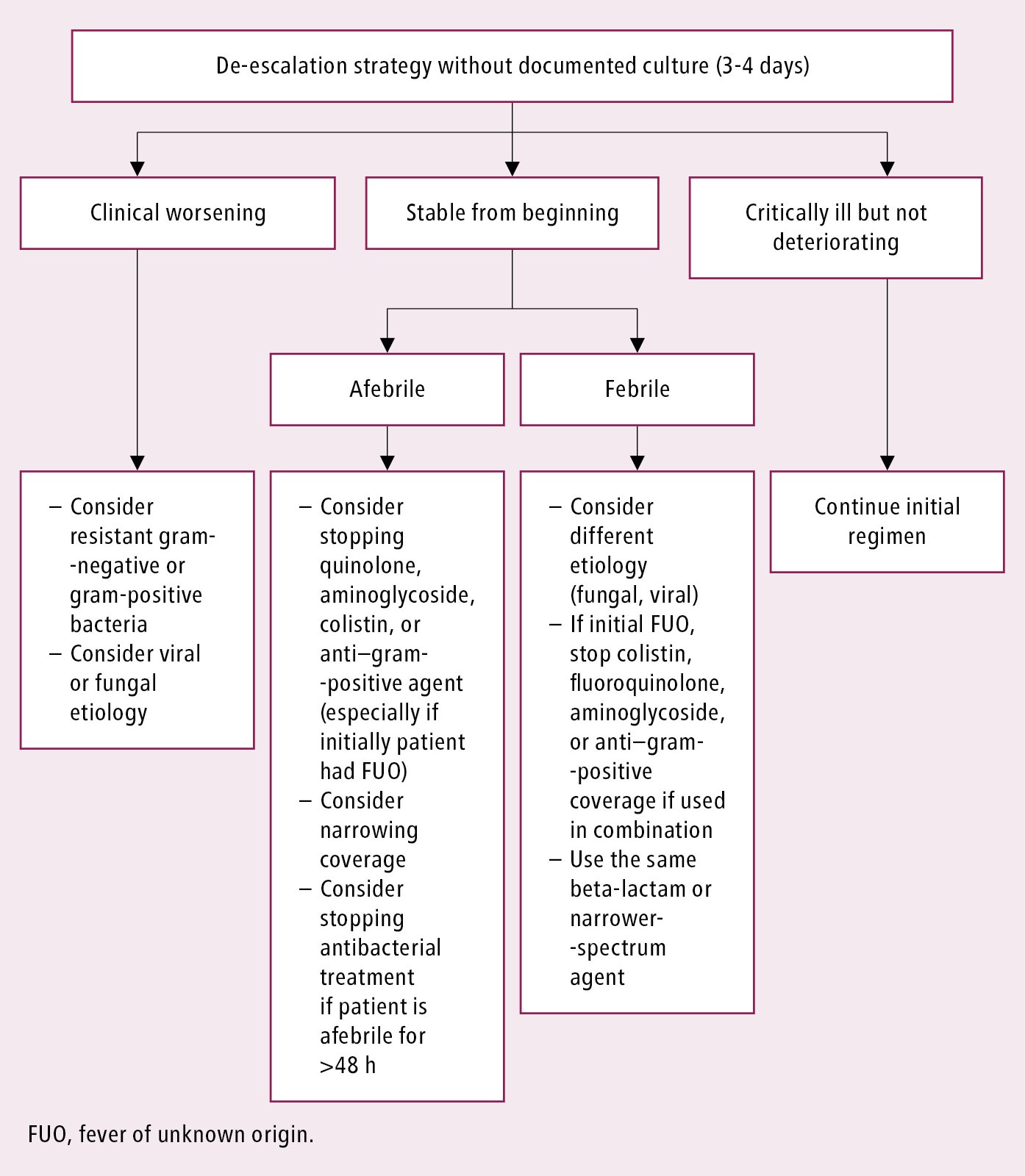
Figure 10.5-2. De-escalation strategy in febrile neutropenia. Adapted from Haematologica. 2013;98(12):1826-35.
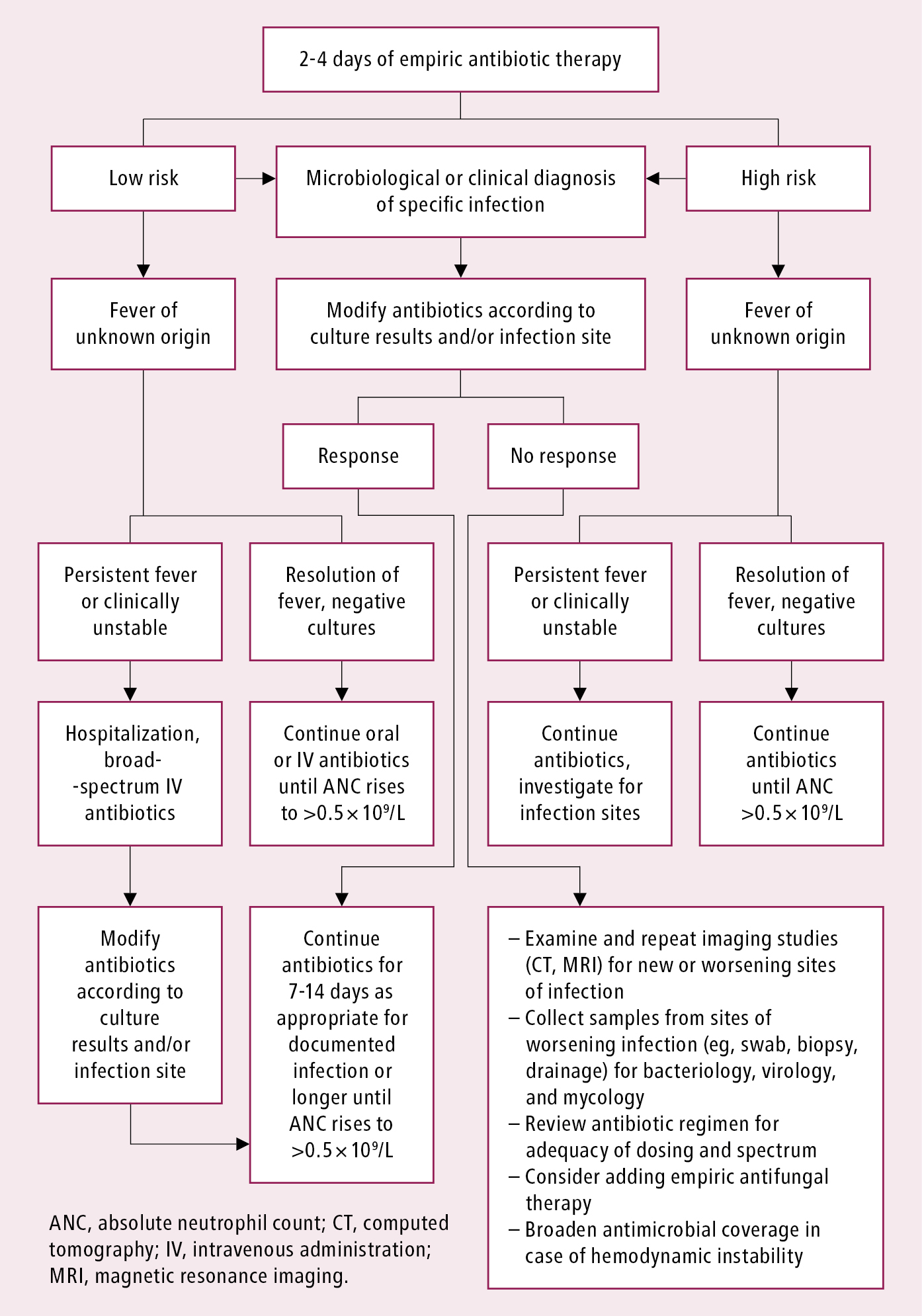
Figure 10.5-3. Reassessment of patients with febrile neutropenia after 2 to 4 days of empiric antibiotic therapy. Source: Clin Infect Dis. 2011;52(4):e56-93.
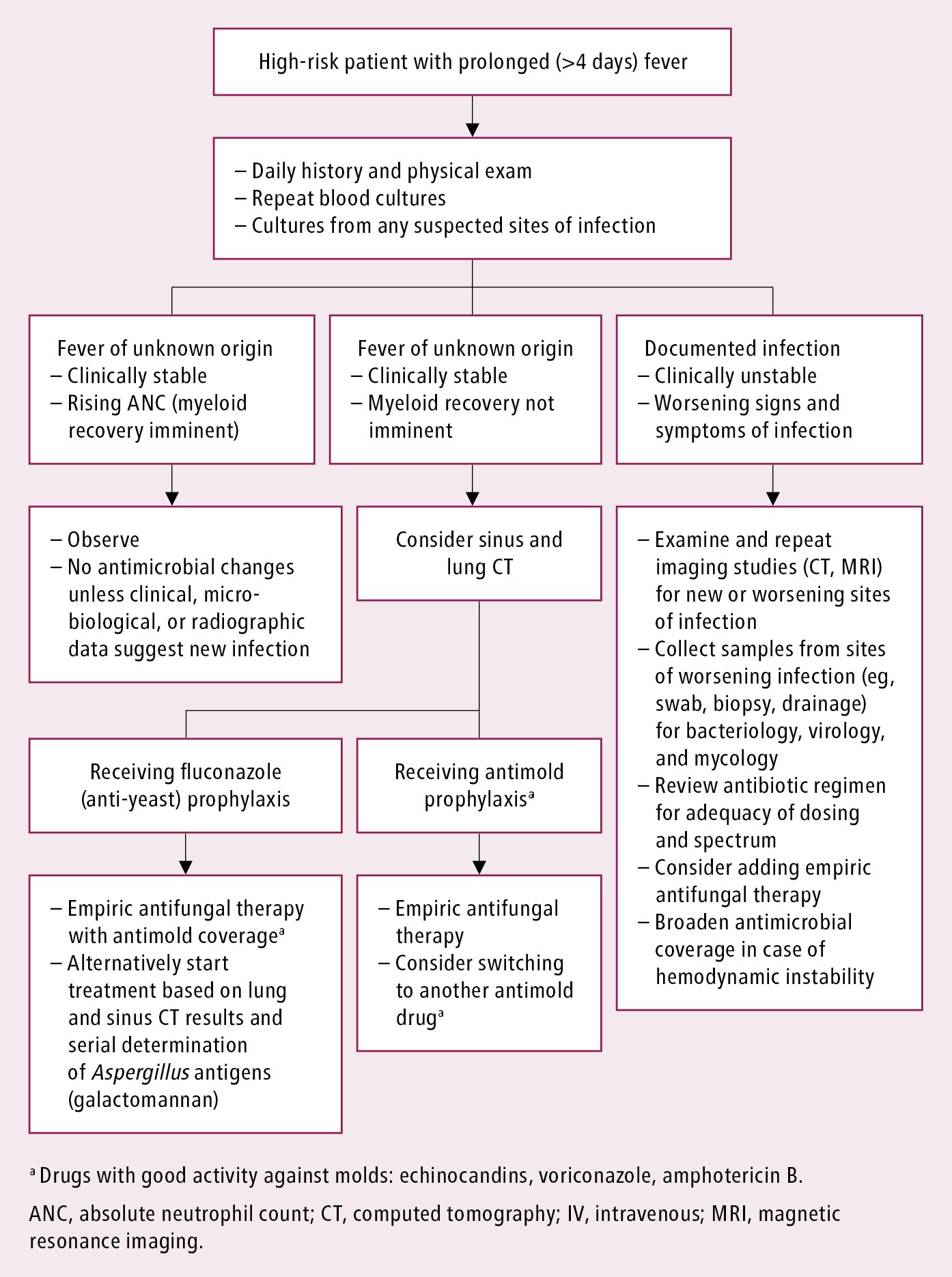
Figure 10.5-4. Management of high-risk patients with fever after 4 days of antibiotic therapy. Source: Clin Infect Dis. 2011;52(4):e56-93.