Shiohara T, Mizukawa Y. Drug-induced hypersensitivity syndrome (DiHS)/drug reaction with eosinophilia and systemic symptoms (DRESS): An update in 2019. Allergol Int. 2019;68(3):301-308. doi: 10.1016/j.alit.2019.03.006. Epub 2019 Apr 16. PMID: 31000444.
Martínez-Cabriales SA, Rodríguez-Bolaños F, Shear NH. Drug Reaction with Eosinophilia and Systemic Symptoms (DReSS): How Far Have We Come? Am J Clin Dermatol. 2019;20(2):217-236. doi: 10.1007/s40257-018-00416-4. PMID: 30652265.
Taweesedt PT, Nordstrom CW, Stoeckel J, Dumic I. Pulmonary manifestations of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome: A systematic review. Biomed Res Int. 2019;2019:7863815. doi: 10.1155/2019/7863815. eCollection 2019. PMID: 31662996; PMCID: PMC6778864.
Watanabe H. Recent advances in drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms. J Immunol Res. 2018;2018:5163129. doi: 10.1155/2018/5163129. eCollection 2018. PMID: 29744372; PMCID: PMC5878892.
Pannu AK, Saroch A. Diagnostic criteria for drug rash and eosinophilia with systemic symptoms. J Family Med Prim Care. Jul-Sep 2017;6(3):693-694. doi: 10.4103/2249-4863.222050. PMID: 29417040; PMCID: PMC5787987.
Kardaun SH, Sidoroff A, Valeyrie-Allanore L, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: Does a DRESS syndrome really exist? Br J Dermatol. 2007;156(3):609-11. doi: 10.1111/j.1365-2133.2006.07704.x. PMID: 17300272.
Definition, Etiology, PathogenesisTop
Drug reaction with eosinophilia and systemic symptoms (DRESS), also known as drug-induced hypersensitivity syndrome (DIHS), is a multiorgan systemic reaction characterized by rash, fever, lymphadenopathy, leukocytosis with eosinophilia and atypical lymphocytes, and liver dysfunction. Drugs commonly implicated to cause DRESS: Table 4.2-1.
The incidence of this syndrome ranges from 1 in 1000 to 1 in 10,000. The disease most often occurs in adults, without sex predilection. The overall mortality rate is 10% to 20%.
The pathogenic factors causing DRESS are not clearly understood, but the hypothesis includes a complex interaction between the drug-specific immune response, viral reactivation (specifically human herpesvirus 6 [HHV-6] and human herpesvirus 7 [HHV-7]), and genetic predisposition in patients carrying specific human leukocyte antigens (HLAs), such as HLA-B*1502, HLA-B*1508, HLA-B*5701, and HLA-B*5801.
Clinical Features and Natural HistoryTop
In most patients the reaction begins within 3 to 8 weeks after the introduction of the culprit drug. Fever and skin rash are the most common initial symptoms.
1. Fever, ranging between 38 degrees Celsius and 40 degrees Celsius, is the most common sign occurring in 90% to 100% of patients.
2. Cutaneous eruption: Skin rash usually involves >50% of the body surface area and may develop into erythroderma. The face, upper trunk, and extremities are usually the initial sites of involvement. Cutaneous eruption is often maculopapular (Figure 4.2-1) but can be urticarial, target-like (Figure 4.2-2), pustular, bullous (Figure 4.2-3), eczematous, or polymorphic. Facial edema is a common presenting sign with fever. Mucosal involvement, mainly of the lips and oral cavity, can be present in >50% of cases.
3. Hematologic abnormalities: Eosinophilia is most common (95%) and often delayed for 1 to 2 weeks. It sometimes occurs after elevated liver enzymes return to baseline. Less frequent abnormalities include neutrophilia (78%), monocytosis (69%), and atypical lymphocytes (67%).
4. Lymphadenopathy occurs in ~50% of patients. It could be generalized or limited and gradually resolves after the withdrawal of the culprit drug.
5. Hepatic involvement occurs in 75% to 90% of patients. The liver is the visceral organ most commonly involved in DRESS. There is no consensus on the definition of liver involvement, but it ranges from mild elevation of liver enzymes to severe fulminant hepatitis. In the scoring system proposed by the Registry of Severe Cutaneous Adverse Reactions (RegiSCAR), liver involvement is defined by the finding of a serum level of alanine aminotransferase (ALT) or bilirubin and/or alkaline phosphatase (ALP) levels that are >2-fold higher than the upper limit of normal on ≥2 different dates. The development of severe hepatitis with jaundice is associated with higher mortality, and hepatic necrosis may cause death in the setting of coagulopathy and sepsis.
6. Renal involvement occurs in up to one-third of patients. It manifests with acute interstitial nephritis with high serum creatinine levels, proteinuria, or pathologic urinary sediment with occasional eosinophils. In the majority of patients, renal involvement is induced by allopurinol.
7. Pulmonary involvement occurs in <30% of patients and can present with a variety of manifestations ranging from mild cough or dyspnea with nonspecific interstitial lesions on chest imaging to acute respiratory distress syndrome (ARDS) with life-threatening hypoxic respiratory failure. Pulmonary symptoms may precede the development of skin eruption or develop later in the course of the disease.
8. Involvement of other organs: Heart (eosinophilic myocarditis, pericarditis), gastrointestinal tract (diarrhea, mucosal erosions, bleeding), pancreas (pancreatitis), thyroid (autoimmune thyroiditis, appearing often late, as a sequela of DRESS), muscle (myositis, increase in creatine kinase levels), eye (uveitis), neurologic system (headaches, seizures, coma, and motor function impairment). Of note, certain drugs have been related to specific organ damage.
Skin rash and involvement of other organs resolve gradually after drug withdrawal; the average time for complete recovery is 6 to 8 weeks. However, there is a small proportion of patients in whom subsequent flare-ups develop after the initial episode and/or the disease persists for several months. Subsequent flare-ups have been reported concurrently with HHV-6 infection reactivation.
DiagnosisTop
The diagnosis of DRESS is mostly based on clinical presentation (fever, skin eruption, and lymphadenopathy) and should be suspected in patients who received a new drug in the previous 8 weeks. Several diagnostic criteria have been proposed. The RegiSCAR group suggested criteria for the diagnosis of DRESS in hospitalized patients. In addition to acute skin rash that is suspected to be drug related, the criteria include fever >38 degrees Celsius, enlarged lymph nodes in ≥2 different body sites, involvement of ≥1 internal organ, and abnormal blood count (lymphocytes above or below the laboratory limits or eosinophils above the laboratory limits, or platelets below the laboratory limits, or atypical lymphocytosis). The presence of ≥3 criteria is required for the diagnosis of DRESS (ncbi.nlm.nih.gov).
The differential diagnosis of DRESS includes Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN), acute generalized exanthematous pustulosis (AGEP), hypereosinophilic syndromes (HES), adult-onset Still disease, severe maculopapular drug eruption, and viral exanthema.
1. Laboratory tests: Complete blood count (CBC) with differential and peripheral blood smear, liver function tests including aspartate aminotransferase (AST), ALT, ALP, bilirubin, serum creatinine and blood urea nitrogen, serum creatine kinase, and urinalysis. The results of these laboratory tests should be frequently monitored during the course of the disease. The frequency should be determined depending on disease severity.
2. Skin biopsy: There are no specific histologic findings for DRESS. However, mild spongiosis and a lymphocytic infiltrate in the superficial dermis, predominantly perivascular, with eosinophils and dermal edema, may support the diagnosis.
3. Imaging studies are indicated in patients with pulmonary-like shortness of breath or cough. A chest radiograph or computed tomography (CT) scan may provide evidence of interstitial pneumonitis or pleural effusion.
4. Patch test or lymphocyte transformation test may help in diagnosis; both tests have good specificity but rather low sensitivity.
TreatmentTop
Identification and prompt withdrawal of the possible offending drug is the mainstay of treatment. Treatment is mainly supportive and symptomatic; systemic treatment is advocated by some authors in situations of severe organ involvement (levels of transaminases >5-fold higher than normal; presence of heart or kidney involvement and hemophagocytosis).
Basic symptomatic management includes topical glucocorticoids, emollients, and oral H1 antihistamines. If signs of severe organ involvement are present, systemic therapy could be considered on the basis of observational data:
1) Systemic glucocorticoids: Oral prednisone 1 mg/kg/d or high-dose methylprednisolone (250-1000 mg IV daily for 3-5 days) are often used. Glucocorticoids are usually given until clinical improvement is observed and laboratory parameters are back to normal limits, and then tapered over 2 to 3 months.
2) Intravenous immunoglobulin (IVIG): Based on observational data of low quality, IVIG can be used in life-threatening contexts, such as hemophagocytosis with bone marrow failure, encephalitis, severe hepatitis, renal failure, and respiratory failure. The dose is often 2 g/kg given over 3 to 5 days. IVIG appears to be associated with higher mortality when given alone in patients with DRESS and therefore should not be administered in the absence of glucocorticoids.
3) Antiviral medications: Ganciclovir can be considered in severe cases and if a major viral reactivation (HHV-6) is confirmed.
4) Other agents such as cyclosporine, mycophenolate mofetil, cyclophosphamide, and rituximab. As case series and case reports have shown the beneficial effect of these drugs, they may be used as a second-line therapy.
Follow-upTop
Patients should be monitored for manifestations of autoimmune diseases, such as hyperthyroidism, hypothyroidism, type 1 diabetes, systemic lupus erythematosus (SLE), autoimmune hemolytic anemia, and sclerodermoid graft-versus-host disease–like lesions. Late flares of DRESS, such as fever and hepatitis, can occur a few weeks after the initial presentation.
PrognosisTop
Visceral involvement resulting in multiorgan failure seems to be unpredictable. However, a higher risk of severe organ involvement has been reported in DRESS induced by allopurinol and minocycline compared with other drugs. Other indicators of poor prognosis include HHV-6 infection reactivation, pancytopenia, hypereosinophilia >1,5×109/L, heart rate >90 beats/min, white blood cells >12×109/L, respiratory rate >20 breaths/min, coagulopathy, and gastrointestinal bleeding. Hepatic dysfunction is the most common cause of death. Cardiac involvement might appear even months after DRESS occurrence and turn into fulminant myocarditis.
Special ConsiderationsTop
Patients who recover from DRESS may be at increased risk of cross-reactions with structurally related and sometimes structurally unrelated drugs, mainly in the first few months. Therefore it is important to educate the patients about the need for a strict avoidance of the offending and cross-reacting drugs. It is also important to counsel these patients about the genetic risks in relatives.
Tables and FiguresTop
|
– Carbamazepine – Phenytoin – Phenobarbital – Zonisamide – Mexiletine – Lamotrigine – Dapsone – Salazosulfapyridine – Allopurinol – Minocycline – Abacavir – Sulfamethoxazole – Nitrofurantoin |
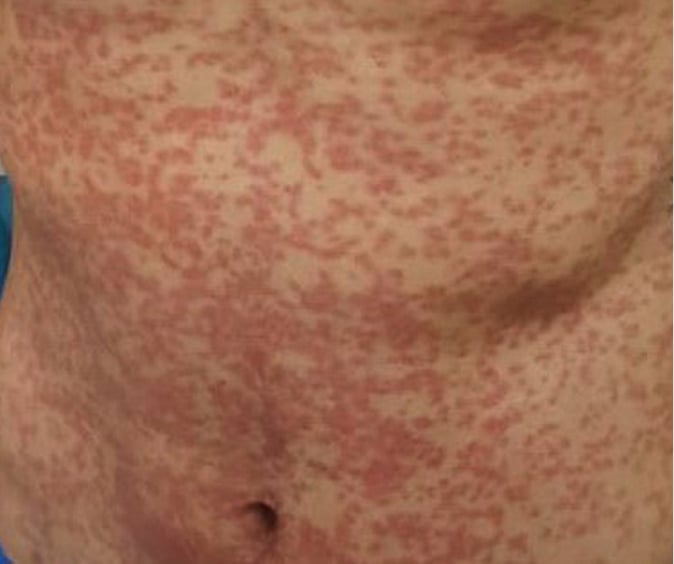
Figure 4.2-1. Drug reaction with eosinophilia and systemic symptoms (DRESS) presenting as a maculopapular rash on the trunk. Photograph courtesy of Dr Hiba Zaaroura.
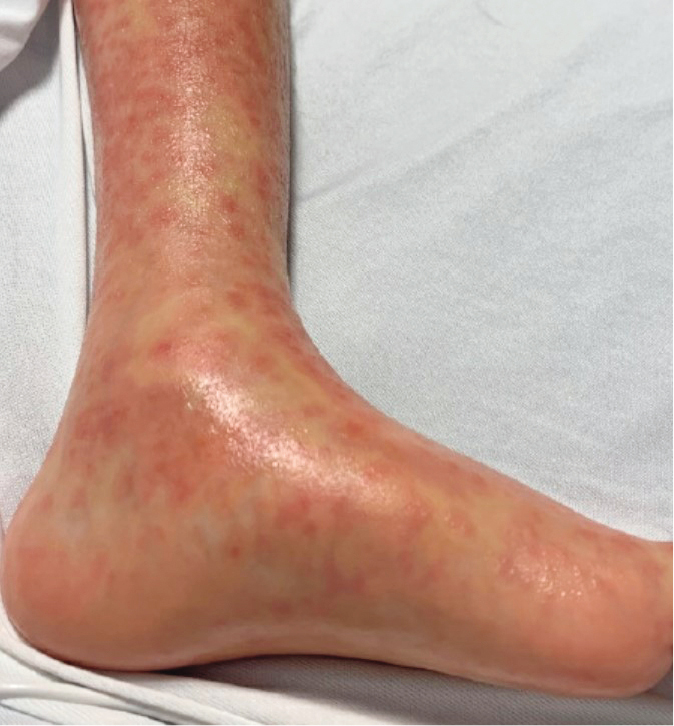
Figure 4.2-2. Drug reaction with eosinophilia and systemic symptoms (DRESS) presenting with target-like lesions on the leg. Photograph courtesy of Dr Hiba Zaaroura.
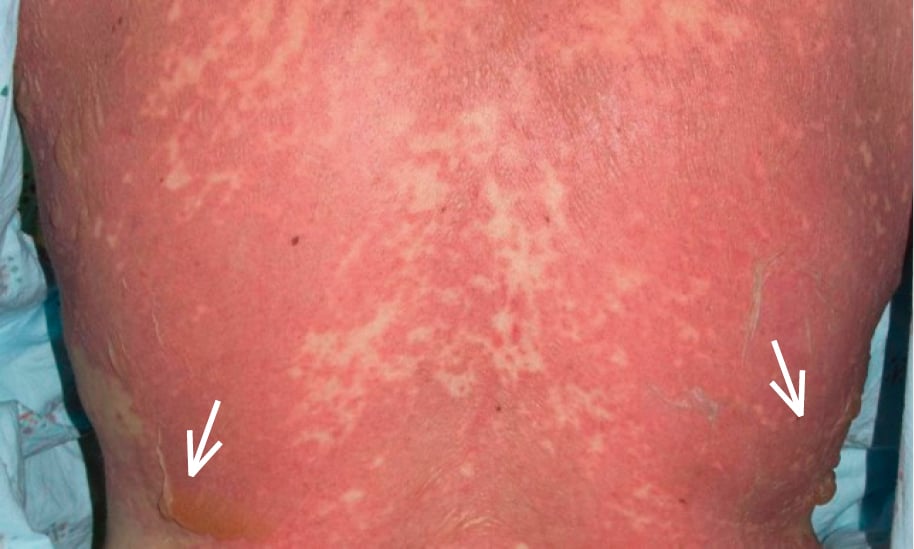
Figure 4.2-3. Multiple bullae on top of erythematous plaques on the back of a patient with drug reaction with eosinophilia and systemic symptoms (DRESS). Photograph courtesy of Dr Neil H. Shear.