Indications Top
1. Diagnostic analysis of pleural effusion:
1) Any new pleural effusion, except in the case of clinically suspected transudate due to heart failure, hypoalbuminemia, cirrhosis, end-stage renal failure, or in patients with small effusions; in such circumstances treat the underlying cause, reassess, and consider thoracentesis if effusion does not resolve with treatment.
2) Persistent effusion despite an adequate trial of therapy of the underlying disease, large unilateral effusions (particularly left-sided), symptoms of pleurisy, dyspnea or fever, or effusion of unknown etiology.
2. Treatment of pleural effusion: Symptomatic lung compression by a pleural effusion. Usually the volumes drained during one procedure should not exceed 1500 mL due to risk of reexpansion pulmonary edema (RPE). Although rare (<1%) and not clearly associated with the volume of fluid removed, the mortality rate of RPE is as high as 20%, which explains the conservative suggestion of drainage of ≤1500 mL at a time.Evidence 1Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). Low Quality of Evidence (low confidence that we know true effects of the intervention). Quality of Evidence lowered due to evidence based only on observational studies. Feller-Kopman D, Berkowitz D, Boiselle P, Ernst A. Large-volume thoracentesis and the risk of reexpansion pulmonary edema. Ann Thorac Surg. 2007 Nov;84(5):1656-61. PubMed PMID: 17954079. Repeat therapeutic thoracentesis may be done several hours later if there is no evidence of RPE and the patient remains symptomatic.
Contraindications Top
1. Absolute: None.
2. Relative: International normalized ratio (INR) >1.5 and activated partial thromboplastin time (aPTT) >2 × upper limit of normal, platelet counts <50×109/L, lack of operator experience, small effusions (increased risk of pneumothorax), or dermatitis/cellulitis at the planned thoracentesis site.
Complications Top
1. Early: Pain at the site of thoracentesis, pneumothorax, hemothorax, visceral injury, vasovagal reflex, procedure failure, adverse effects of local anesthetics and disinfectants.
2. Late: Skin infection at the site of thoracentesis, empyema and dissemination of cancer cells along the needle track (particularly in mesothelioma).
Patient Preparation Top
1. Obtain informed consent. The optimal patient position is sitting with arms supported (Figure 1).
2. Tests: Review a recent chest radiograph; if available, use pleural ultrasonography immediately before or during thoracentesis (this is associated with lower failure rates and lower complication rates).Evidence 2Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). Moderate Quality of Evidence (moderate confidence that we know true effects of intervention). Quality of Evidence lowered due to the observational nature of most studies, and increased due to a large effect (odds ratio, 0.3; 95% confidence interval, 0.2-0.7); on balance, QoE is moderate. Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010 Feb 22;170(4):332-9. doi: 10.1001/archinternmed.2009.548. Review. PubMed PMID: 20177035.
Equipment Top
1. Surgical field preparation (see Surgical Field Preparation for Small Procedures) and local anesthesia (see Infiltration Anesthesia).
2. Needle (bore 0.8 mm [21 gauge]) and a syringe (50-60 mL) in diagnostic thoracentesis. In the case of anticipated collection of higher fluid volumes, use a special thoracentesis kit including a syringe, large-bore thoracentesis catheter (bore 1.4-2 mm [18-14 gauge]) fitted with an aspiration needle, 3-way stopcock, pressure tubing, and bottle for collecting effusion fluid.
3. Syringes and tubes for fluid samples (as required depending on indications):
1) Chemistry (basic: protein, lactate dehydrogenase, pH, glucose, albumin [compared with serum values]; as needed: triglycerides, total cholesterol, amylase, adenosine deaminase): Dry tube, 2 to 5 mL of fluid.
2) Cell count and differential count: Dry ethylenediaminetetraacetic acid (EDTA) or heparinized tube, 2 to 3 mL of fluid. Hematocrit can be measured if a hemothorax is suspected (pleural fluid hematocrit >50% of the patient’s peripheral blood hematocrit).
3) Cytology: Heparinized tube (1 mL), >30 to 50 mL of fluid. Fluid can be sent for flow cytometry if malignancy is suspected.
4) Microbiology: Sterile plastic container or transport culture medium. If infection is suspected, pleural fluid should additionally be sent in blood culture bottles (this increases diagnostic yield). If empyema is suspected a specific anaerobic culture medium should be prepared and pleural fluid should be immediately put into the medium (with tight seal) to minimize air exposure, which could result in lower yield of anaerobe growth. Acid-fast bacilli and tuberculosis culture should be performed when there is clinical suspicion of mycobacterial pleuritis.
Site of Thoracentesis Top
Use bedside pleural ultrasonography for guidance, if available (Figure 2). It is common practice to proceed with pleural fluid aspiration with the patient in the sitting position and the needle inserted posteriorly (Figure 1). Palpate the intercostal space; using an aseptic technique, perform thoracentesis at the ultrasonography-guided site at the superior margin of a rib to reduce the risk of injury to the neurovascular bundle. A lateral site is preferred using an ultrasonography-guided technique as the risk of intercostal vessel trauma increases with more posterior or medial procedures. In the case of large free-flowing effusions, the percussion method can be used to identify the thoracentesis site (2 intercostal spaces below the upper limit of the dull percussion area) if ultrasonography is not available. Using this approach, the needle is inserted equidistant from the spinous process and the posterior axillary line.
Of note, the neurovascular bundle may not be entirely covered by the rib in this position and the triangle of safety (bordered anteriorly by the lateral edge of pectoralis major, laterally by the lateral edge of latissimus dorsi, inferiorly by the line of the fifth intercostal space, and superiorly by the base of the axilla) is considered a safer alternative by the British Thoracic Society.
Procedure Top
1. Use ultrasonography, if available, to mark the thoracentesis site.
2. Prepare the surgical field (see Surgical Field Preparation for Small Procedures); use sterile technique.
3. Perform infiltration anesthesia of the skin, subcutaneous tissues, and parietal pleura using 1% lidocaine without epinephrine (5-10 mL is usually sufficient; see Infiltration Anesthesia).
4. Advance the thoracentesis needle, perpendicularly to the skin, with an overlying catheter. If one is available, towards the pleural effusion along the ultrasound-marked site (Figure 3) while aspirating (constantly drawing back the syringe plunger).
5. After the fluid appears in the needle, advance the catheter further into the pleural cavity while withdrawing the needle during expiration (patient can be asked to hum during this process), then disconnect the syringe. Connect the catheter to a special kit or a 3-way stopcock draining into a bottle or a bag; if a needle without a catheter is used, connect it to a 50 or 60 mL syringe before performing thoracentesis.
6. Collect fluid samples in syringes and tubes.
After the Procedure Top
Withdraw the needle or catheter (this is best done on expiration) and secure the site of thoracentesis using a small sterile dressing.
FiguresTop
Figure 21.17-1. Patient position during thoracentesis.
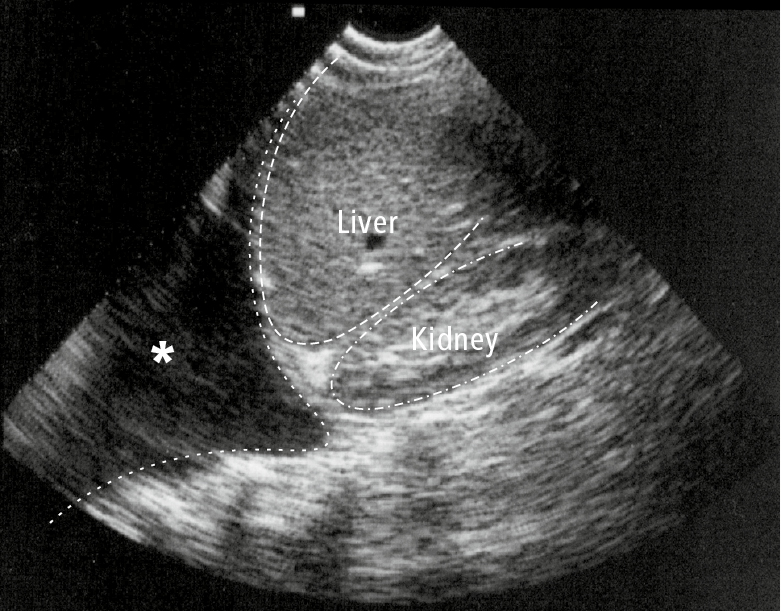
Figure 21.17-2. Ultrasonography of the right hypochondrium visualizing a pleural effusion (asterisk).
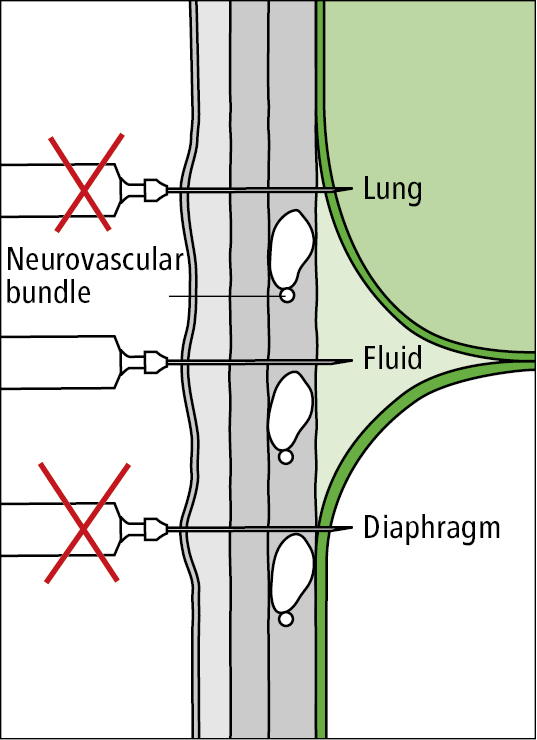
Figure 21.17-3. Appropriate thoracentesis approach.