Los trastornos de la coagulación, típicos para las personas con hipotiroidismo clínico, pueden manifestarse en mujeres en forma de menstruación abundante. Las demás manifestaciones del sistema reproductor en mujeres incluyen ciclos anovulatorios, causados por la alteración de la conversión de los precursores de estrógeno. El hipotiroidismo también causa alteraciones en el sistema urinario, como disminución de la filtración glomerular, y en especial la alteración en la excreción de agua libre, que como efecto puede conducir a la aparición de hiponatremia. En cuanto al sistema nervioso central, a menudo se observa el cansancio, la depresión, la pérdida de la memoria y la incapacidad para concentrarse. El estadio final del hipotiroidismo grave es el coma en el curso de mixedema.40 El término "encefalopatía de Hashimoto" describe una patología muy rara, que se caracteriza por la presencia de anticuerpos anti-TPO, niveles elevados de proteína en el líquido cefalorraquídeo, lesiones corticales inespecíficas en la resonancia magnética y una respuesta variable a la terapia con glucocorticoides.41
Diagnóstico
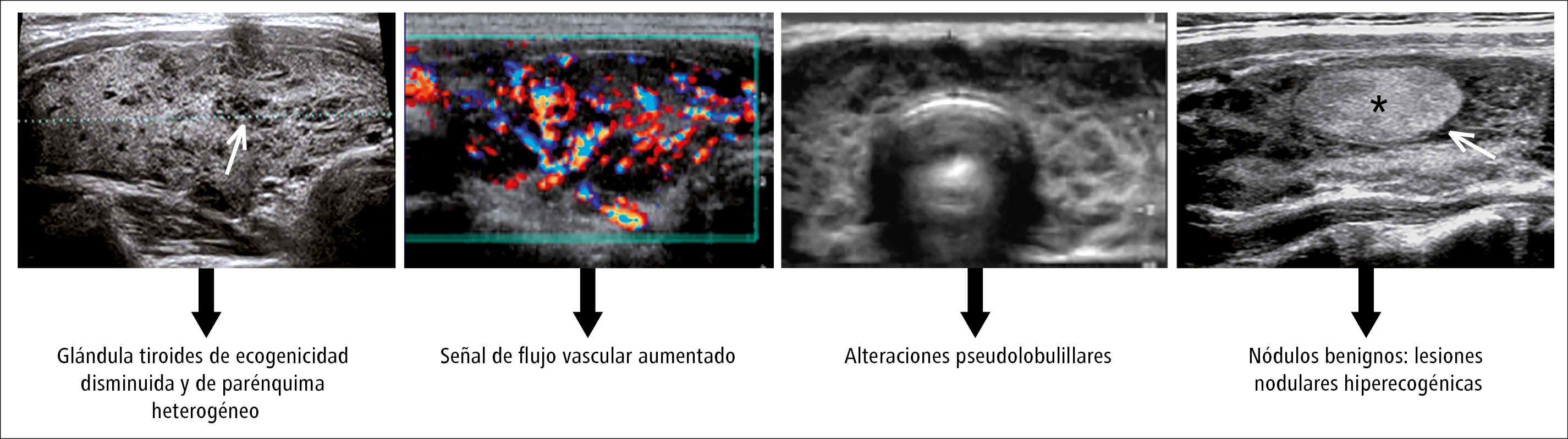
El diagnóstico de la enfermedad de Hashimoto se basa en la observación de manifestaciones clínicas de hipotiroidismo y la presencia de anticuerpos anti-TPO (sin embargo, se debe tener en cuenta la forma seronegativa de la enfermedad de Hashimoto, que se presenta en un 5-10 % de los casos). En el diagnóstico diferencial, especialmente en pacientes seronegativos, puede ser útil la ecografía de tiroides.37 La ecogenicidad disminuida de la glándula tiroides, la heterogeneidad del parénquima, el flujo vascular aumentado y la presencia de los pequeños quistes son las características ecográficas típicas de la enfermedad de Hashimoto (fig. 3).42
Los anticuerpos anti-TPO se observan en el suero en un 95 % de los enfermos, mientras que los anticuerpos anti-Tg en un 60-80 %.41,43 Los anticuerpos anti-TPO se consideran un factor de riesgo de progresión de la enfermedad de Hashimoto al hipotiroidismo clínico, tanto en población general, como en las personas que desarrollaron el hipotiroidismo tras la exposición a amiodarona, litio o interferón α. Los anticuerpos anti-TPO se asocian al riesgo de desarrollo de hipotiroidismo durante el embarazo, el riesgo elevado de aborto espontáneo y del fracaso de la fecundación in vitro.44
Fig. 3. Imagen ecográfica de la glándula tiroides en la enfermedad de Hashimoto
Volver al artículo principal: Guías: enfermedad de Hashimoto. Etiología, diagnóstico y tratamiento
Bibliografía:
- Hashimoto H., The knowledge of the lymphomatous changes in the thyroid gland (struma lymphomatosa) [en alemán], Archiv für klinische Chirurgie, 1912; 97: 219
- Caturegli P., De Remigis A., Chuang K. y cols., Hashimoto’s thyroiditis: celebrating the centennial through the lens of the Johns Hopkins hospital surgical pathology records, Thyroid, 2013; 23: 142-150
- Ralli M., Angeletti D., Fiore M. y cols., Hashimoto’s thyroiditis: an update on pathogenic mechanisms, diagnostic protocols, therapeutic strategies, and potential malignant transformation, Autoimmun. Rev., 2020; 19: 102 649
- McLeod D.S., Caturegli P., Cooper D.S. y cols., Variation in rates of autoimmune thyroid disease by race/ethnicity in US military personnel, JAMA, 2014; 311: 1563-1565
- Song R.H., Yao Q.M., Wang B. y cols., Thyroid disorders in patients with myasthenia gravis: a systematic review and meta analysis, Autoimmun. Rev., 2019; 18: 102 368
- Yao Q., Song Z., Wang B. y cols., Thyroid disorders in patients with sys temic sclerosis: a systematic review and meta analysis, Autoimmun. Rev., 2019; 18: 634-636
- Nakamura H., Usa T., Motomura M. y cols., Prevalence of interrelated auto antibodies in thyroid diseases and autoimmune disorders, J. Endocrinol. Ivest., 2008; 31: 861-865
- Feldt Rasmussen U., Hoier Madsen M., Bech K. y cols., Anti thyroid peroxidase antibodies in thyroid disorders and non thyroid autoimmune diseases, Autoimmunity, 1991; 9: 245-254
- Bliddal S., Nielsen C.H., Feldt Rasmussen U., Recent advances in understanding autoimmune thyroid disease: the tallest tree in the forest of polyautoimmunity, F1000Research, 2017; 6: 1776
- Lazúrová I., Benhatchi K., Autoimmune thyroid diseases and nonorgan specific autoimmunity, Pol. Arch. Med. Wewn., 2012; 122, suppl. 1: 55-59
- Eisenbarth G.S., Gottlieb P.A., Autoimmune polyendocrine syndromes, N. Engl. J. Med., 2004; 350: 2068-2079
- Brix T.H., Hegedüs L., Twin studies as a model for exploring the aetiology of autoimmune thyroid disease, Clin. Endocrinol., 2012; 76: 457-464
- Gleicher N., Barad D.H., Gender as risk factor for autoimmune diseases, J. Autoimmun., 2007; 28: 1-6
- Brand O., Gough S., Heward J., HLA, CTLA 4 and PTPN22: the shared genetic master key to autoimmunity?, Expert Rev. Mol. Med., 2005; 7: 1-15
- Weetman A.P., The immunopathogenesis of chronic autoimmune thyroiditis one century after hashimoto, Eur. Thyroid J., 2013; 1: 243-250
- Johar A., Sarmiento Monroy J.C., Rojas Villarraga A. y cols., Definition of mutations in polyautoimmunity, J. Autoimmun., 2016; 72: 65-72
- Santos L.R., Duraes C., Mendes A. y cols., A polymorphism in the promoter region of the selenoprotein S gene (SEPS1) contributes to Hashimoto’s thyroiditis susceptibility, J. Clin. Endocrinol. Metab., 2014; 99: E719-723
- Jia X., Wang B., Yao Q. y cols., Variations in CD14 gene are associated with autoimmune thyroid diseases in the Chinese population, Front. Endocrinol., 2018; 9: 811
- Feil R., Fraga M.F., Epigenetics and the environment: emerging patterns and implications, Nat. Rev. Genet., 2012; 13: 97-109
- Lepez T., Vandewoestyne M., Deforce D., Fetal microchimeric cells in autoimmune thyroid diseases: harmful, beneficial or innocent for the thyroid gland?, Chimerism, 2013; 4: 111-118
- Mynster Kronborg T., Frohnert Hansen J., Nielsen C.H. y cols., Effects of the commercial flame retardant mixture DE 71 on cytokine production by human immune cells, PloS One, 2016; 11: e0154621
- Wiersinga W.M., Clinical relevance of environmental factors in the pathogenesis of autoimmune thyroid disease, Endocrinol. Metab. (Seoul), 2016; 31: 213-222
- Gong B., Wang C., Meng F. y cols., Association between gut microbiota and autoimmune thyroid disease: a systematic review and meta analysis, Front. Endocrinol., 2021; 12: 774362
- Aghini Lombardi F., Fiore E., Tonacchera M. y cols., The effect of voluntary iodine prophylaxis in a small rural community: the Pescopagano survey 15 years later, J. Clin. Endocrinol. Metab., 2013; 98: 1031-1039
- Carayanniotis G., Recognition of thyroglobulin by T cells: the role of iodine, Thyroid, 2007; 17: 963-973
- Alexander E.K., Pearce E.N., Brent G.A. y cols., 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum, Thyroid, 2017; 27: 315-389
- Toulis K.A., Anastasilakis A.D., Tzellos T.G. y cols., Selenium supplementation in the treatment of Hashimoto’s thyroiditis: a systematic review and a meta analysis, Thyroid, 2010; 20: 1163-1173
- Winther K.H., Wichman J.E., Bonnema S.J. y cols., Insufficient documentation for clinical efficacy of selenium supplementation in chronic autoimmune thyroiditis, based on a systematic review and meta analysis, Endocrine, 2017; 55: 376-385
- Metso S., Hyytiä Ilmonen H., Kaukinen K. y cols., Gluten free diet and auto immune thyroiditis in patients with celiac disease. A prospective controlled study, Scand. J. Gastroenterol., 2012; 47: 43-48
- Krysiak R., Szkrobka W., Okopień B., The effect of gluten free diet on thyroid autoimmunity in drug naive women with hashimoto’s thyroiditis: a pilot study, Exp. Clin. Endocrinol., 2019; 127: 417-422
- Giordano C., Stassi G., De Maria R. y cols., Potential involvement of Fas and its ligand in the pathogenesis of Hashimoto’s thyroiditis, Science, 1997; 275: 960-963
- Ahmed R., Al Shaikh S., Akhtar M., Hashimoto thyroiditis: a century later, Adv. Anat. Pathol., 2012; 19: 181-186
- Hennessey J.V., Clinical review: Riedel’s thyroiditis: a clinical review, J. Clin. Endocrinol. Metab., 2011; 96: 3031-3041
- Stone J.H., Khosroshahi A., Deshpande V. y cols., Recommendations for the nomenclature of IgG4 related disease and its individual organ system manifestations, Arthritis Rheum., 2012; 64: 3061-3067
- Zhang Q.Y., Ye X.P., Zhou Z. y cols., Lymphocyte infiltration and thyrocyte destruction are driven by stromal and immune cell components in Hashimoto’s thyroiditis, Nat. Commun., 2022; 13: 775
- Pedersen I.B., Knudsen N., Jorgensen T. y cols., Thyroid peroxidase and thyroglobulin autoantibodies in a large survey of populations with mild and moderate iodine deficiency, Clin. Endocrinol., 2003; 58: 36-42
- Jonklaas J., Bianco A.C., Bauer A.J. y cols., Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement, Thyroid, 2014; 24: 1670-1751
- Biondi B., Cappola A.R., Cooper D.S., Subclinical hypothyroidism: a review, JAMA, 2019; 322: 153-160
- Boucai L., Hollowell J.G., Surks M.I., An approach for development of age, gender, and ethnicity specific thyrotropin reference limits, Thyroid, 2011; 21: 5-11
- Jonklaas J., Optimal thyroid hormone replacement, Endocr. Rev, 2022; 43: 366-404
- Caturegli P., De Remigis A., Rose N.R., Hashimoto thyroiditis: clinical and diagnostic criteria, Autoimmun. Rev., 2014; 13: 391-397
- Anderson L., Middleton W.D., Teefey S.A. y cols., Hashimoto thyroiditis: part 2, sonographic analysis of benign and malignant nodules in patients with diffuse Hashimoto thyroiditis, AJR Am. J. Roentgenol., 2010; 195: 216-222
- McLachlan S.M., Rapoport B., Why measure thyroglobulin autoantibodies rather than thyroid peroxidase autoantibodies?, Thyroid, 2004; 14: 510-520
- Jonklaas J., Bianco A.C., Cappola A.R. y cols., Evidence based use of levothyroxine/liothyronine combinations in treating hypothyroidism: a consensus document, Eur. Thyroid J., 2021; 10: 10-38
- Fatourechi V., McConahey W.M., Woolner L.B., Hyperthyroidism associated with histologic Hashimoto’s thyroiditis, Mayo Clin. Proc., 1971; 46: 682-689
- Rodondi N., den Elzen W.P., Bauer D.C. y cols., Subclinical hypothyroidism and the risk of coronary heart disease and mortality, JAMA, 2010; 304: 1365-1374
- Chaker L., Baumgartner C., den Elzen W.P. y cols., Subclinical hypothyroidism and the risk of stroke events and fatal stroke: an individual participant data analysis, J. Clin. Endocrinol. Metab., 2015; 100: 2181-2191
- Kabadi U.M., Jackson T., Serum thyrotropin in primary hypothyroidism. A possible predictor of optimal daily levothyroxine dose in primary hypothyroidism, Arch. Intern. Med., 1995; 155: 1046-1048
- Lillevang Johansen M., Abrahamsen B., Jorgensen H.L. y cols., Duration of over and under treatment of hypothyroidism is associated with increased cardiovascular risk, Eur. J. Endocrinol., 2019; 180: 407-416
- Huang H.K., Wang J.H., Kao S.L., Association of hypothyroidism with all cause mortality: a cohort study in an older adult population, J. Clin. Endocrinol. Metab., 2018; 103: 3310-3318
- Lillevang Johansen M., Abrahamsen B., Jorgensen H.L. y cols., Over and under treatment of hypothyroidism is associated with excess mortality: a register based cohort study, Thyroid, 2018; 28: 566-574
- Thayakaran R., Adderley N.J., Sainsbury C. y cols., Thyroid replacement therapy, thyroid stimulating hormone concentrations, and long term health outcomes in patients with hypothyroidism: longitudinal study, BMJ, 2019; 366: 4892
- Okosieme O.E., Belludi G., Spittle K. y cols., Adequacy of thyroid hormone replacement in a general population, QJM – Mon. J. Assoc. Physicians, 2011; 104: 395-401
- Ettleson M.D., Bianco A.C., Zhu M. y cols., Sociodemographic disparities in the treatment of hypothyroidism: NHANES 2007–2012, J. Endocr. Soc., 2021; 5: bvab041
- Santini F., Pinchera A., Marsili A. y cols., Lean body mass is a major determinant of levothyroxine dosage in the treatment of thyroid diseases, J. Clin. Endocrinol. Metab., 2005; 90: 124-127
- Pilo A., Iervasi G., Vitek F. y cols., Thyroidal and peripheral production of 3,5,3’ triiodothyronine in humans by multicompartmental analysis, Am. J. Physiol., 1990; 258: E715 726
- Devdhar M., Drooger R., Pehlivanova M. y cols., Levothyroxine replacement doses are affected by gender and weight, but not age, Thyroid, 2011; 21: 821-827
- Jonklaas J., Sex and age differences in levothyroxine dosage requirement, Endocr. Pract., 2010; 16: 71-79
- Hollowell J.G., Staehling N.W., Flanders W.D. y cols., Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III), J. Clin. Endocrinol. Metab., 2002; 87: 489-499
- Taylor P.N., Razvi S., Pearce S.H. y cols., Clinical review: a review of the clinical consequences of variation in thyroid function within the reference range, J. Clin. Endocrinol. Metab., 2013; 98: 3562-3571
- Samuels M.H., Kolobova I., Niederhausen M. y cols., Effects of altering levothyroxine (LT4) doses on quality of life, mood, and cognition in LT4 treated subjects, J. Clin. Endocrinol. Metab., 2018; 103: 1997-2008
- Samuels M.H., Kolobova I., Niederhausen M. y cols., Effects of altering levothyroxine dose on energy expenditure and body composition in subjects treated with LT4, J. Clin. Endocrinol. Metab., 2018; 103: 4163-4175
- Walsh J.P., Ward L.C., Burke V. y cols., Small changes in thyroxine dosage do not produce measurable changes in hypothyroid symptoms, well being, or quality of life: results of a double blind, randomized clinical trial, J. Clin. Endocrinol. Metab., 2006; 91: 2624-2630
- Boeving A., Paz Filho G., Radominski R.B. y cols., Low normal or high normal thyrotropin target levels during treatment of hypothyroidism: a prospective, comparative study, Thyroid, 2011; 21: 355-360
- Sturgess I., Thomas S.H., Pennell D.J. y cols., Diurnal variation in TSH and free thyroid hormones in patients on thyroxine replacement, Acta Endocrinol., 1989; 121: 674-676
- Massolt E.T., Meima M.E., Swagemakers S.M.A. y cols., Thyroid state regulates gene expression in human whole blood, J. Clin. Endocrinol. Metab., 2018; 103: 169-178
- Nygaard B., Jensen E.W., Kvetny J. y cols., Effect of combination therapy with thyroxine (T4) and 3,5,3’-triiodothyronine versus T4 monotherapy in patients with hypothyroidism, a double-blind, randomised cross-over study, Eur. J. Endocrinol., 2009; 161: 895-902
- Bunevicius R., Kazanavicius G., Zalinkevicius R. y cols., Effects of thyroxine as compared with thyroxine plus triiodothyronine in patients with hypothyroidism, N. Eng. J. Med., 1999; 340: 424-429
- Escobar Morreale H.F., Botella Carretero J.I., Gomez Bueno M. y cols., Thyroid hormone replacement therapy in primary hypothyroidism: a randomized trial comparing L thyroxine plus liothyronine with L thyroxine alone, Ann. Intern. Med., 2005; 142: 412-424
- Saravanan P., Simmons D.J., Greenwood R. y cols., Partial substitution of thyroxine (T4) with triiodothyronine in patients on T4 replacement therapy: results of a large community based randomized controlled trial, J. Clin. Endocrinol. Metab., 2005; 90: 805-812
- Valizadeh M., Seyyed Majidi M.R., Hajibeigloo H. y cols., Efficacy of combined levothyroxine and liothyronine as compared with levothyroxine monotherapy in primary hypothyroidism: a randomized controlled trial, Endocr. Res., 2009; 34: 80-89
- Ma C., Xie J., Huang X. y cols., Thyroxine alone or thyroxine plus triiodothyronine replacement therapy for hypothyroidism, Nucl. Med. Commun., 2009; 30: 586-593
- Escobar Morreale H.F., Botella Carretero J.I., Morreale de Escobar G.: Treatment of hypothyroidism with levothyroxine or a combination of levo thyroxine plus L triiodothyronine, Best. Pract. Res. Clin. Endocrinol. Metab., 2015; 29: 57-75
- Joffe R.T., Brimacombe M., Levitt A.J. y cols., Treatment of clinical hypothyroidism with thyroxine and triiodothyronine: a literature review and meta analysis, Psychosomatics, 2007; 48: 379-384
- Grozinsky Glasberg S., Fraser A., Nahshoni E. y cols., Thyroxine triiodothyronine combination therapy versus thyroxine monotherapy for clinical hypothyroidism: meta analysis of randomized controlled trials, J. Clin. Endocrinol. Metab., 2006; 91: 2592-2599
- Celi F.S., Zemskova M., Linderman J.D. y cols., The pharmacodynamic equivalence of levothyroxine and liothyronine: a randomized, double blind, cross over study in thyroidectomized patients, Clin. Endocrinol., 2010; 72: 709-715
- Shakir M.K.M., Brooks D.I., McAninch E.A. y cols., Comparative effectiveness of levothyroxine, desiccated thyroid extract, and levothyroxine + liothyronine in hypothyroidism, J. Clin. Endocrinol. Metab., 2021; 106: e4400-e4413
- Castagna M.G., Dentice M., Cantara S. y cols., DIO2 Thr92Ala reduces deiodinase 2 activity and serum T3 levels in thyroid deficient patients, J. Clin. Endocrinol. Metab., 2017; 102: 1623-1630
- Panicker V., Saravanan P., Vaidya B. y cols., Common variation in the DIO2 gene predicts baseline psychological well being and response to combination thyroxine plus triiodothyronine therapy in hypothyroid patients, J. Clin. Endocrinol. Metab., 2009; 94: 1623-1629
- Carle A., Faber J., Steffensen R. y cols., Hypothyroid patients encoding combined MCT10 and DIO2 gene polymorphisms may prefer L T3 + L T4 combination treatment – data using a blind, randomized, clinical study, Eur. Thyroid J., 2017; 6: 143-151
- Wouters H.J., van Loon H.C., van der Klauw M.M. y cols., No effect of the Thr92Ala polymorphism of deiodinase 2 on thyroid hormone parameters, health related quality of life, and cognitive functioning in a large population based cohort study. Thyroid, 2017; 27: 147–155
- Young Cho Y., Jeong Kim H., Won Jang H. y cols., The relationship of 19 functional polymorphisms in iodothyronine deiodinase and psychological well being in hypothyroid patients, Endocrine, 2017; 57: 115-124
- Appelhof B.C., Fliers E., Wekking E.M. y cols., Combined therapy with levothyroxine and liothyronine in two ratios, compared with levothyroxine monotherapy in primary hypothyroidism: a double blind, randomized, controlled clinical trial, J. Clin. Endocrinol. Metab., 2005; 90: 2666-2674
- Gan T., Randle R.W.: The role of surgery in autoimmune conditions of the thyroid, Surg. Clin. North Am., 2019; 99: 633-648
- Guldvog I., Reitsma L.C., Johnsen L. y cols., Thyroidectomy versus medical management for euthyroid patients with hashimoto disease and persisting symptoms: a randomized trial, Ann. Intern. Med., 2019; 170: 453-464
- Jankovic B., Le K.T., Hershman J.M.: Clinical Review: Hashimoto’s thyroiditis and papillary thyroid carcinoma: is there a correlation?, J. Clin. Endocrinol. Metab., 2013; 98: 474-482
- Sulaieva O., Selezniov O., Shapochka D. y cols., Hashimoto’s thyroiditis attenuates progression of papillary thyroid carcinoma: deciphering immunological links, Heliyon, 2020; 6: e03 077
- Abbasgholizadeh P., Naseri A., Nasiri E. y cols., Is Hashimoto thyroiditis associated with increasing risk of thyroid malignancies? A systematic review and meta analysis, Thyroid Res., 2021; 14: 26
- Haugen B.R., Alexander E.K., Bible K.C. y cols., 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer, Thyroid, 2
- De Leo S., Pearce E.N.: Autoimmune thyroid disease during pregnancy, Lancet Diabetes Endocrinol., 2018; 6: 575-586
- Thangaratinam S., Tan A., Knox E. y cols., Association between thyroid autoantibodies and miscarriage and preterm birth: meta-analysis of evidence, BMJ, 2011; 342: d2616
- Xie J., Jiang L., Sadhukhan A. y cols., Effect of antithyroid antibodies on women with recurrent miscarriage: a metaanalysis, Am. J. Reprod. Immunol, 2020; 83: e13 238
- Liu H., Shan Z., Li C. y cols., Maternal subclinical hypothyroidism, thyroid autoimmunity, and the risk of miscarriage: a prospective cohort study, Thyroid, 2014; 24: 1642-1649
- Korevaar T.I.M., Derakhshan A., Taylor P.N. y cols., Association of thyroid function test abnormalities and thyroid autoimmunity with preterm birth: a systematic review and meta-analysis, JAMA, 2019; 322: 632-641
- Lee S.Y., Pearce E.N., Assessment and treatment of thyroid disorders in pregnancy and the postpartum period, Nat. Rev. Endocrinol., 2022; 18: 158-171
- Korevaar T.I., Schalekamp Timmermans S., de Rijke Y.B. y cols., Hypothyroxinemia and TPO antibody positivity are risk factors for premature delivery: the generation R study, J. Clin. Endocrinol. Metab., 2013; 98: 4382-4390
- Thompson W., Russell G., Baragwanath G. y cols., Maternal thyroid hormone insufficiency during pregnancy and risk of neurodevelopmental disorders in offspring: a systematic review and meta analysis, Clin. Endocrinol., 2018; 88: 575-584
- Davis L.E., Leveno K.J., Cunningham F.G.: Hypothyroidism complicating pregnancy, Obstet. Gynecol., 1988; 72: 108-112
- Leung A.S., Millar L.K., Koonings P.P. y cols., Perinatal outcome in hypothyroid pregnancies, Obstet. Gynecol., 1993; 81: 349-353
- Mannisto T., Mendola P., Grewal J. y cols., Thyroid diseases and adverse pregnancy outcomes in a contemporary US cohort, J. Clin. Endocrinol. Metab., 2013; 98: 2725-2733
- Okosieme O.E., Khan I., Taylor P.N.: Preconception management of thyroid dysfunction, Clin. Endocrinol., 2018; 89: 269-279
- Calvo R., Obregon M.J., Ruiz de Ona C. y cols., Congenital hypothyroidism, as studied in rats. Crucial role of maternal thyroxine but not of 3,5,3’ tri iodothyronine in the protection of the fetal brain, J. Clin. Invest., 1990; 86: 889-899
- Lau L., Benham J.L., Lemieux P. y cols., Impact of levothyroxine in women with positive thyroid antibodies on pregnancy outcomes: a systematic review and meta analysis of randomised controlled trials, BMJ Open, 2021; 11: e043 751
- Ma R., Morshed S.A., Latif R. y cols., Thyroid cell differentiation from murine induced pluripotent stem cells, Front. Endocrinol., 2015; 6: 56
- Antonica F., Kasprzyk D.F., Opitz R. y cols., Generation of functional thyroid from embryonic stem cells, Nature, 2012; 491: 66-71
 Español
Español
 English
English
 українська
українська