Definition and EtiologyTop
Hirsutism refers to excessive male-pattern hair growth in androgen-dependent areas, such as the upper lip, chest, abdomen, back, buttock, and inner thighs, where women typically have little to no hair. It may be idiopathic, caused by androgen excess, or drug induced.
Virilization occurs with a more severe androgen excess and includes hirsutism, male-pattern hair loss (starting above the temple and visible also around the top of the head), acne, deepening of the voice, increased muscle mass, reduced breasts and uterus size, and clitoromegaly.
Hypertrichosis refers to generalized excessive hair growth not limited to locations sensitive to androgens and not caused by hyperandrogenemia. It may be hereditary, idiopathic, or drug induced (phenytoin, penicillamine, diazoxide, minoxidil, cyclosporine [INN ciclosporin]). It also occurs in women with hypothyroidism, anorexia nervosa, porphyria, or dermatomyositis.
Causes of androgen excess in women:
1) Ovarian dysfunction: Polycystic ovary syndrome (PCOS) (most common), androgen-secreting ovarian tumor, hyperthecosis (hyperplasia of the theca interna of the ovary leading to increased production of androgens).
2) Adrenal gland dysfunction: Androgen-secreting adrenal tumor, Cushing syndrome, congenital adrenal hyperplasia caused by 21-hydroxylase or 11beta-hydroxylase deficiency.
3) Other endocrinopathies: Hyperprolactinemia, acromegaly, insulin resistance.
4) Drugs: Androgens, anabolic steroids, danazol, oral contraceptives containing androgenic progestogens, valproic acid.
5) Idiopathic hirsutism.
DiagnosisTop
Take a medication history as well as history of dysmenorrhea and galactorrhea. Establish the time of onset and rate of progression of hirsutism (sudden-onset, rapidly developing hirsutism not coinciding with puberty and virilization may suggest an ovarian or adrenal tumor, which may be malignant). Assess the type and distribution of hair in all androgen-sensitive locations; look for the features of Cushing syndrome). In patients with PCOS hirsutism may be associated with menstrual irregularities and obesity with insulin resistance.
Hair distribution and type are indicators of the degree of androgen excess. Hirsutism may be assessed with the Ferriman-Gallwey score (Figure 1). This score considers 9 androgen-sensitive areas. The criteria for identifying hirsutism are arbitrary and vary among different ethnic groups, from 2 in Asian through 9 in White to 10 in the Mediterranean populations, although scores much lower than 9 may be associated with androgen excess.
Diagnostic algorithm for hirsutism: Figure 2.
ManagementTop
Address the underlying cause. Discontinue any potentially responsible medications if possible.
Assess the impact of hirsutism on the patient and whether treatment is desired.
If the patient has PCOS, assess for associated conditions, such as metabolic risk factors (obesity, insulin resistance, impaired glucose tolerance [IGT], dyslipidemia) and anovulatory infertility. Counsel on lifestyle changes including diet and weight loss, as this may decrease insulin resistance, lower serum androgen levels, and improve fertility. Consider metformin in patients with obesity, insulin resistance, and IGT or impaired fasting glucose (IFG). Referral to specialists who regularly deal with these conditions (eg, endocrinologists, gynecologists, infertility specialists) may be needed.
First-line pharmacologic therapy for hirsutism is an oral estrogen-progestin contraceptive if the risk for venous thromboembolism is considered acceptable. If oral contraceptives are contraindicated or response to this therapy is not adequate after 6 months, spironolactone may be considered as monotherapy or in combination therapy. Patients should be counseled on the teratogenic effects of spironolactone in pregnancy and need for adequate contraception.
Consider direct hair removal methods for mild hirsutism with no underlying endocrinopathies.
FiguresTop
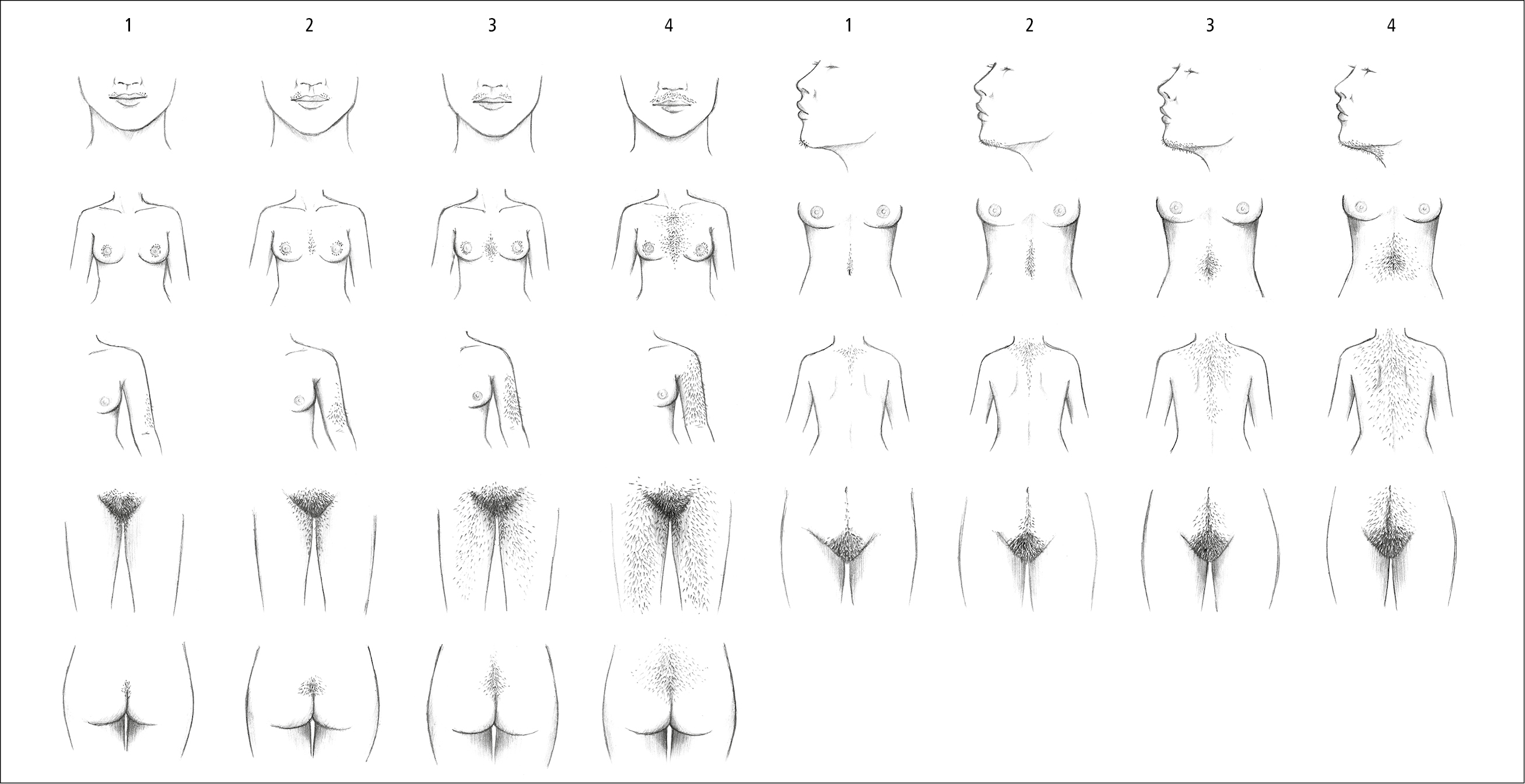
Figure 1.20-1. Ferriman-Gallwey score. Illustration courtesy of Dr Shannon Zhang.
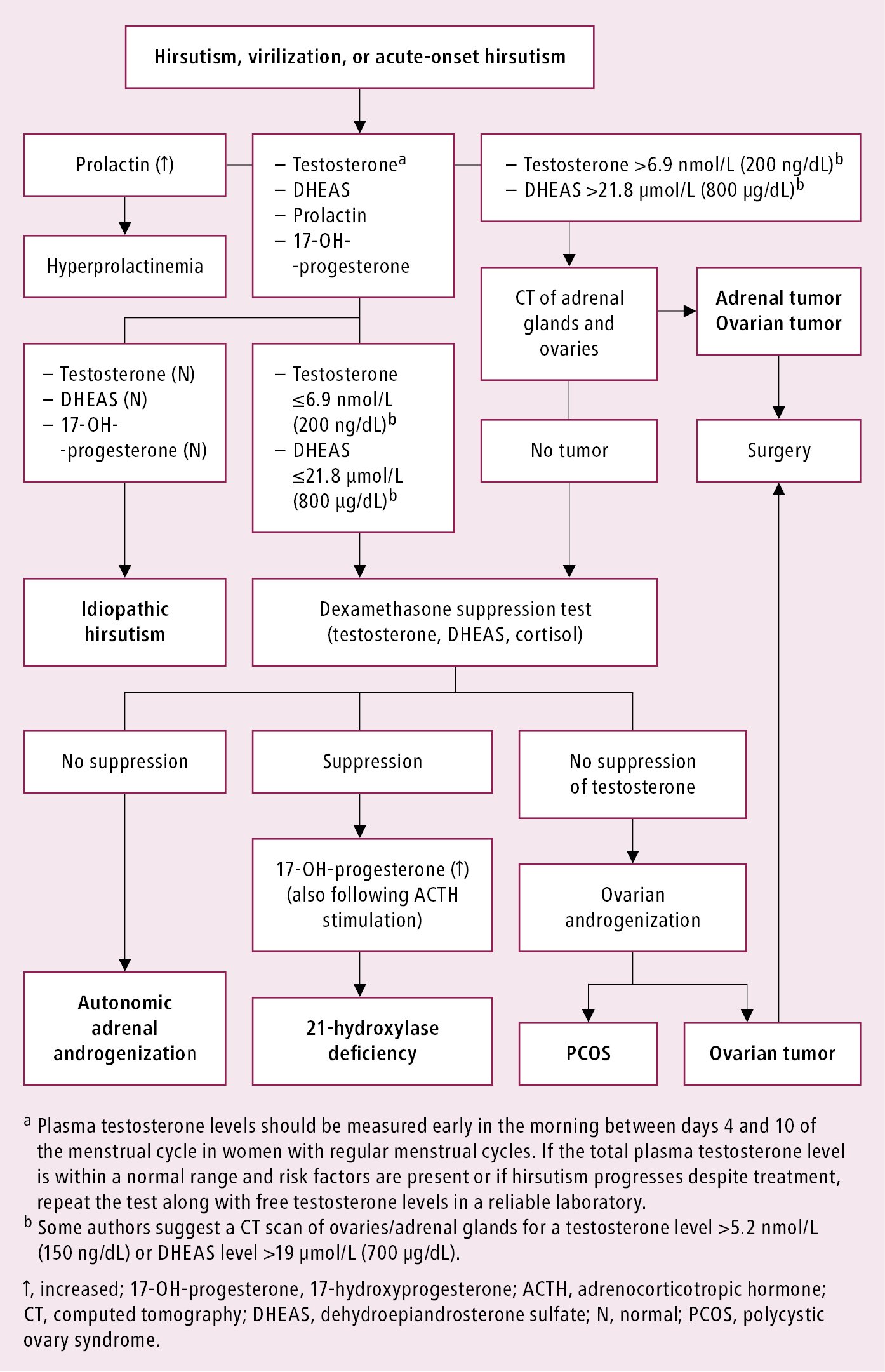
Figure 1.20-2. Diagnostic algorithm for hirsutism and virilization.