American Diabetes Association. Standards of Medical Care in Diabetes - 2021. Diabetes Care 2021;43(suppl 1):S1-S222.
Araszkiewicz A, Bandurska-Stankiewicz E, Budzyński A, et al. 2019 Guidelines on the management of diabetic patients. A position of Diabetes Poland. Clinical Diabetology. 2019;8(1):1-95. doi: 10.5603/DK.2019.0001.
Diabetes Canada Clinical Practice Guidelines Expert Committee. Diabetes Canada 2018 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Can J Diabetes. 2018;42(suppl 1):S1-S325.
Chamberlain JJ, Rhinehart AS, Shaefer CF Jr, Neuman A. Diagnosis and Management of Diabetes: Synopsis of the 2016 American Diabetes Association Standards of Medical Care in Diabetes. Ann Intern Med. 2016 Apr 19;164(8):542-52. doi: 10.7326/M15-3016. Epub 2016 Mar 1. PubMed PMID: 26928912.
World Health Organization. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy. Published 2013. Accessed August 1, 2017.
Blumer I, Hadar E, Hadden DR, et al. Diabetes and pregnancy: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2013 Nov;98(11):4227-49. doi: 10.1210/jc.2013-2465. PubMed PMID: 24194617.
Definition, Etiology, PathogenesisTop
Gestational diabetes mellitus (GDM) describes diabetes mellitus (DM) diagnosed in the second or third trimester of pregnancy in patients who meet ≥1 of the appropriate diagnostic criteria without clear diagnosis of overt diabetes prior to that time. Experts differ slightly in their views on optimal strategies for the diagnosis of GDM (Figure 6.2-1).
Risk factors include multiparity, age >35 years, previous delivery of a child >4000 g of birth weight, delivery of a child with malformations, intrauterine death, hypertension or a body mass index (BMI) >30 kg/m2 before pregnancy, high-risk ethnic groups (eg, Hispanic, African American, Arab, South Asian, or Indigenous populations), family history of type 2 DM, or history of GDM (in ~30% of patients GDM develops again in a subsequent pregnancy).
DiagnosisTop
Diabetes Canada (DC) suggests measuring fasting plasma glucose (FPG) and glycated hemoglobin (HbA1c) levels at the first office visit in all pregnant women who have the risk factors for GDM mentioned above.
If FPG and HbA1c are within the reference range (<5.6 mmol/L and <6.0%, respectively) at the first visit, preferably in the first trimester, in all pregnant women within 24 to 28 weeks of pregnancy the DC suggests performing:
1) One-step diagnostic oral glucose tolerance test (OGTT) (fasting, with the administration of 75 g of glucose); or
2) Two-step OGTT, which starts with a nonfasting 50-g glucose challenge test. If the result is ≥7.8 mmol/L (140 mg/dL) at 1 hour, then a fasting 2-hour 75-g OGTT is required.
GDM is diagnosed in patients with ≥1 abnormal glucose level found in either the 1-step or 2-step test (Figure 6.2-1).
If the HbA1c is ≥6.5% or the FPG is ≥7.0 mmol/L, the woman should be considered to have diabetes in pregnancy and the same management recommendations as for preexisting diabetes should be followed.
All women should be screened for diabetes between 6 weeks and 6 months post partum with a 75-g OGTT using standard diabetes criteria (see Diabetes Mellitus).
See Figure 6.2-1.
TreatmentTop
1. Start with nutritional therapy, ideally before 15 weeks of gestation, in consultation with a registered dietitian. There is a paucity of evidence that one diet compared with others improves maternal or fetal outcomes.Evidence 1Weak recommendation (downsides likely outweigh benefits, but the balance is close or uncertain; an alternative course of action may be better for some patients). Low Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due the risk of bias, imprecision, and indirectness to patient-important outcomes. Han S, Crowther CA, Middleton P, Heatley E. Different types of dietary advice for women with gestational diabetes mellitus. Cochrane Database Syst Rev. 2013 Mar 28;(3):CD009275. doi: 10.1002/14651858.CD009275.pub2. Review. Update in: Cochrane Database Syst Rev. 2017 Feb 25;2:CD009275. PubMed PMID: 23543574. However, ~80% of pregnant patients with GDM can be controlled solely with an adequate diet. The daily caloric intake depends on the prepregnancy BMI, physical activity, and term of pregnancy:
1) Underweight: 40 kcal/kg/d.
2) Normal weight: 30 kcal/kg/d.
3) Overweight and obesity: 20 to 25 kcal/kg/d.
4) Morbid obesity (BMI ≥40 kg/m2): 12 to 14 kcal/kg/d.
The suggested diet may include 35% to 45% of carbohydrates (mainly complex carbohydrates and low–glycemic index foods), 25% to 35% of fats (with equal proportions of saturated and unsaturated fats), and 20% of protein (1.3 g/kg/d). It is recommended to substitute a percentage of animal protein with nonanimal protein (eg, broccoli, mushrooms). A high fiber daily intake (≥20 g/1000 kcal) is suggested. It is also recommended to consume 3 moderate-sized meals in addition to 2 to 4 snacks, including an evening snack.
The Dietary Reference Intake (DRI) suggests a minimal consumption of 175 g of carbohydrates, 71 g of proteins, and 24 g of fiber. The design of nutritional therapy has to be individualized in each patient and it is highly recommended that the assessment be done by a dietitian with expertise in GDM. The goal of this treatment is to have an adequate balance in caloric intake that benefits both the offspring and the mother and to achieve adequate glycemic control.
Physical activity in women with GDM should be encouraged unless obstetric contraindications exist, as it may be an important component of GDM management.
2. Pharmacologic therapy: In patients who have been compliant with appropriate nutritional therapy and physical activity for 7 to 14 days and have not achieved normal blood glucose levels (criteria for glycemic control: see Diabetes Mellitus in Pregnancy) or those with an initial highly elevated glucose level, insulin is suggested as the first-line therapy. Intensive insulin therapy (a multiple daily injection regimen) using subcutaneous injections of short-acting human insulin or a rapid-acting insulin analogue and intermediate-acting human insulin (insulin isophane [NPH]) can be started. Although glargine and detemir have primarily been assessed in women with preexisting diabetes in pregnancy, randomized trial evidence suggests that detemir (basal analogue) is safe and may afford less maternal hypoglycemia compared with NPH, while observational studies suggest that glargine is also safe. Oral glucose-lowering drugs were previously considered to be contraindicated; however, metformin and glyburide (alone or in combination with insulin) have been proven effective and safe in patients with GDM and can also be used if the patient declines to use insulin. Metformin, compared with other therapeutic options like insulin or sulfonylureas, has been associated with smaller weight gain and lower prevalence of pregnancy-induced hypertension and neonatal hypoglycemia; it crosses the placental barrier. Glyburide can also be used during pregnancy in those who decline to use insulin and do not tolerate or are inadequately controlled on metformin, but it has been associated with increased prevalence of neonatal hypoglycemia (fetal hyperinsulinism) and birth weight ≥4000 g.Evidence 2Weak recommendation (downsides likely outweigh benefits, but the balance is close or uncertain; an alternative course of action may be better for some patients). Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Balsells M, García-Patterson A, Solà I, Roqué M, Gich I, Corcoy R. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ. 2015 Jan 21;350:h102. doi: 10.1136/bmj.h102. Review. PubMed PMID: 25609400; PubMed Central PMCID: PMC4301599.
To improve pregnancy outcomes, patients with GDM should check capillary blood glucose levels, both fasting and postprandial. The target blood glucose values are the same as in pregnant patients with preexisting diabetes: fasting and preprandial <5.3 mmol/L, 1-hour postprandial <7.8 mmol/L, 2-hour postprandial <6.7 mmol/L, and >3.7 mmol/L if on insulin to avoid hypoglycemia. Pharmacotherapy should be added if the above-specified glycemic targets are not being met with nutritional therapy and physical activity within 1 to 2 weeks.
3. Antepartum monitoring: GDM is associated with increased perinatal mortality, especially in women with poor glycemic control during the antepartum period. There is an increased risk of macrosomia, stillbirths, shoulder dystocia, and caesarean sections. While there is a paucity of evidence in this area, a few randomized controlled trials and observational studies have shown reduction in shoulder dystocia but increased risk of neonatal hyperbilirubinemia with induction of labor as opposed to expectant management after 38 weeks. Therefore, DC recommends weekly fetal assessments starting at 34 to 36 weeks, using a nonstress test, biophysical profile, or amniotic fluid index. Induction of labor before 40 weeks of gestational age should be individualized, weighing the benefits against the risk of neonatal complications.
4. Management during delivery: In patients treated with insulin, the management during delivery is the same as in preexisting DM and aims to keep glucose levels at 4.0 to 7.0 mmol/L to decrease neonatal hypoglycemia (see Diabetes Mellitus in Pregnancy). In women who have achieved satisfactory blood glucose control with diet alone, insulin should be administered during delivery only if blood glucose levels are >7.0 mmol/L.
5. Management after delivery: Any type of glucose-lowering therapy should be discontinued immediately unless there is a high suspicion for type 1 DM. Since patients with GDM are at increased risk for developing type 2 DM, a 2-hour 75-g OGTT is recommended between 6 weeks and 6 months post partum. Patients meeting the criteria for prediabetes should be counseled about their increased risk of DM and should consider lifestyle modification strategies (nutritional therapy, metformin, and exercise). In patients diagnosed with DM, start lifestyle changes and oral medications as in any other patient with a recent diagnosis of type 2 DM. Women with GDM should be encouraged to breastfeed immediately after birth and for a minimum of 4 months to decrease the risk of neonatal hypoglycemia, childhood obesity, diabetes in both the mother and child, and hypertension in the mother.
FIGURESTop
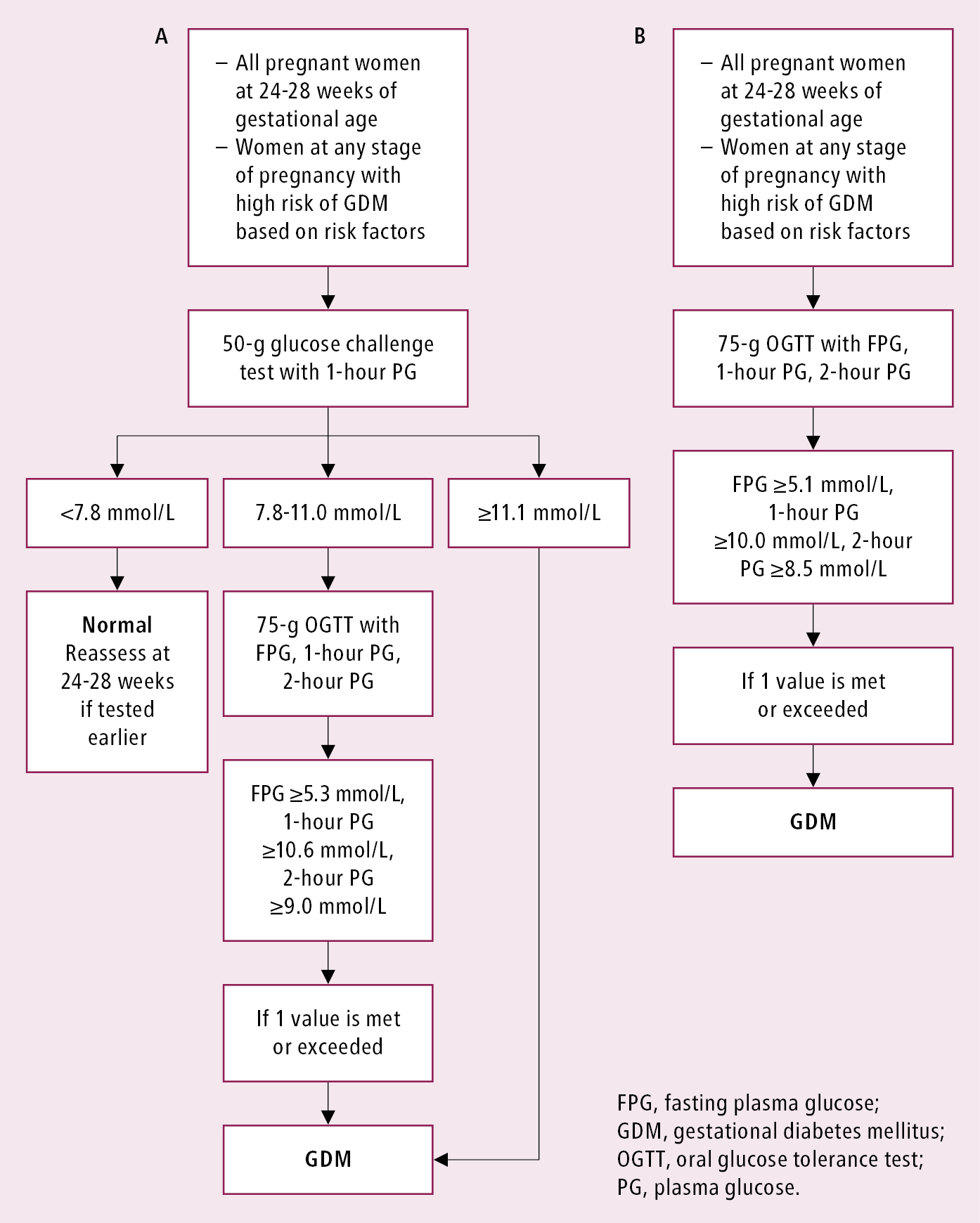
Figure 6.2-1. Diagnostic criteria for GDM (2 approaches). A, the preferred approach as per Diabetes Canada, using a 50-g glucose challenge test as an initial screen and a 75-g OGTT as the confirmatory step. B, the alternative approach that can also be used for diagnosis. Adapted from Can J Diabetes. 2018;42(suppl 1):S255–S282.