Mercado MG, Smith DK, Guard EL. Acute Kidney Injury: Diagnosis and Management. Am Fam Physician. 2019 Dec 1;100(11):687-694. PMID: 31790176.
Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney inter., Suppl. 2012; 2: 1–138.
Definition, Etiology, PathogenesisTop
Acute kidney injury (AKI) is a clinical syndrome arbitrarily defined by either an abrupt increase in serum creatinine concentration by ≥26.5 micromol/L (0.3 mg/dL) within 48 hours, a ≥1.5-fold increase in serum creatinine over the prior 7 days, or urine output <0.5 mL/kg/h for 6 hours (Table 1). This definition, as per the latest Kidney Disease: Improving Global Outcomes (KDIGO) 2012 consensus guidelines, takes into account the RIFLE (risk, injury, failure; loss, end-stage renal disease) criteria created by the Acute Dialysis Quality Initiative (ADQI) and the Acute Kidney Injury Network (AKIN) definition. It also highlights the increased mortality resulting from even small increases in creatinine.
The yearly incidence of AKI is about 200 per 1,000,000 patients, occurring in 5% of hospitalized patients and 30% of patients in intensive care units. The occurrence of AKI is linked to increased mortality. Age, sex, and medical comorbidities are risk factors for the development of AKI, with older men being at the highest risk.
The diagnostic approach is broadly divided into 3 groups: prerenal, intrinsic (renal), and postrenal (Figure 1).
1. Prerenal AKI is characterized by a decrease in glomerular filtration rate (GFR) in response to impaired renal perfusion with intact renal parenchyma. However, intact tubular function with high urine osmolality and low urine sodium concentrations should not necessarily be interpreted as prerenal AKI, as many intrinsic etiologies, such as glomerulonephritis or AKI due to sepsis, may initially have intact tubular function. Etiology:
1) Decreased effective circulatory volume (hypovolemia):
a) Hemorrhage: Traumatic, surgical, postpartum, gastrointestinal GI).
b) GI fluid loss: Vomiting, diarrhea, surgical drainage.
c) Kidney loss: Diuretic therapy, osmotic diuresis in diabetes, and adrenal insufficiency.
d) Vasodilatory loss of the extravascular compartments: Sepsis syndromes, acute pancreatitis, peritonitis, severe trauma, burns, and severe hypoalbuminemia.
2) Reduced cardiac output: Diseases of the myocardium, valves, and pericardium; arrhythmias; massive pulmonary embolism; positive–pressure mechanical ventilation.
3) Vascular abnormalities:
a) Vasodilation: Sepsis, hypotension caused by antihypertensive medications (including drugs that reduce afterload), and general anesthesia.
b) Vasoconstriction: Hypercalcemia, norepinephrine, epinephrine, tacrolimus, cyclosporine (INN cyclosporin), and amphotericin B.
4) Impaired renal autoregulation and hypoperfusion: Cyclooxygenase inhibitors (nonsteroidal anti-inflammatory drugs [NSAIDs]), angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), and direct renin inhibitors.
5) Hyperviscosity syndrome: Multiple myeloma, Waldenström macroglobulinemia, and polycythemia.
6) Renal vessel occlusion:
a) Renal artery occlusion: Atherosclerosis, thromboembolism, dissecting aneurysm, and systemic vasculitis.
b) Renal vein occlusion: Thromboembolism and external compression.
2. Intrinsic AKI is characterized by a decrease in GFR with loss of renal parenchymal integrity due to both inflammatory and noninflammatory factors. Etiology:
1) Small vessel disease: Thrombotic microangiopathy (TMA) (hemolytic–uremic syndrome [HUS], thrombotic thrombocytopenic purpura [TTP]), cholesterol crystal embolization, disseminated intravascular coagulation (DIC), preeclampsia or eclampsia, malignant hypertension, systemic lupus erythematosus (SLE), and progressive systemic sclerosis (scleroderma renal crisis).
2) Acute tubular necrosis (ATN):
a) Ischemia: Prolonged prerenal AKI.
b) Exogenous toxins: Radiographic contrast agents, cyclosporine, antibiotics (eg, aminoglycosides), chemotherapy (cisplatin), ethylene glycol, methanol, and NSAIDs.
c) Endogenous toxins: Myoglobin, hemoglobin, monoclonal proteins (eg, in multiple myeloma).
d) Crystals: Uric acid, oxalic acid (a metabolite of ethylene glycol), acyclovir (INN aciclovir) (particularly IV), methotrexate, sulfonamides, and indinavir.
3) Acute interstitial nephritis (AIN):
a) Allergic: Beta–lactam antibiotics, sulfonamides, trimethoprim, rifampin (INN rifampicin), NSAIDs, diuretics, captopril, and proton pump inhibitors.
b) Infectious: Bacterial (legionella, leptospirosis), viral (cytomegalovirus, BK virus), and fungal (candidiasis).
c) Infiltrative: Malignant (lymphoma, leukemia), granulomatous (sarcoidosis), and idiopathic.
d) Autoimmune: Sjögren syndrome, tubulointerstitial nephritis with uveitis (TINU) syndrome, IgG4-related kidney disease (IgG4-RKD), SLE.
4) Glomerulonephritis (GN):
a) Anti–glomerular basement membrane (GBM) disease (sometimes referred to as Goodpasture syndrome or disease).
b) Antineutrophil cytoplasmic antibody (ANCA)-associated GN (granulomatosis with polyangiitis [formerly Wegener granulomatosis], eosinophilic granulomatosis with polyangiitis [formerly Churg-Strauss syndrome], microscopic polyangiitis).
c) Immune complex–mediated GN (SLE, postinfectious, cryoglobulinemia, and primary membranoproliferative GN).
5) Acute graft rejection following renal transplant.
6) Other rare causes: Acute renal cortical necrosis, Chinese herb (aristolochic acid) nephropathy, acute phosphate nephropathy, warfarin-related nephropathy, loss of the solitary functioning kidney.
3. Postrenal AKI or obstructive nephropathy develops in the case of obstructed urinary flow from either structural or functional impediment in the urinary tract. Etiology:
1) Obstruction of either both ureters or a single ureter in a solitary functioning kidney:
a) Occlusion: Calculi, blood clots, sloughed renal papillae.
b) External compression: A tumor or retroperitoneal fibrosis.
c) Damage: Inadvertent ligation or incision during surgical procedures.
2) Diseases of the bladder: Neurogenic bladder, bladder neck obstruction by a tumor (bladder cancer), calculi, blood clots.
3) Diseases of the prostate: Benign prostatic hypertrophy or cancer.
4) Urethral disease: Obstruction by a foreign body or calculi and trauma.
Clinical Features and Natural HistoryTop
The clinical presentation of AKI varies depending on the cause, severity, and associated diseases related to renal injury. Most patients with mild to moderate AKI are asymptomatic and identified by laboratory testing. Patients with severe AKI may have symptoms of uremia including fatigue, loss of appetite, weight loss, pruritus, nausea, vomiting, muscle cramps, and changes in mental status.
Oliguria or anuria occurs in ~50% of patients and is frequently seen with prerenal AKI, acute renal cortical necrosis, thromboembolism, and thrombotic microangiopathy. Normal or even increased urine output can be seen in intrinsic etiologies of AKI.
Historically, 4 phases were distinguished in the natural course of AKI (it is now recognized that some presentations of AKI are nonoliguric and do not conform to this description):
1) Initiation phase: This phase presents with normal urine output as it commences from the initial impact of the insult (cause) until the point of actual kidney damage. The duration of this phase is usually several hours and varies depending on the causative factor.
2) Oliguria (urine output 100-400 mL/d) or anuria (urine output <100 mL/d): This phase occurs when urine output is typically between 50 and 400 mL/d. It develops in ~50% of patients and lasts an average of 10 to 14 days but can vary from 1 day to 8 weeks.
3) Polyuria: This phase begins with rapidly increasing urine output over several days after a period of oliguria or anuria. It occurs due to tubular dysfunction and is manifested by sodium wasting and polyuria. Serum creatinine and urea levels may not decrease for several days. The duration of polyuria is proportional to the duration of oliguria/anuria and may last up to several weeks. This phase of AKI is associated with considerable risk of dehydration and severe loss of electrolytes, particularly potassium and calcium.
4) Recovery phase: During this phase urine output gradually returns to normal and serum creatinine and urea begin to normalize. It may take up to several months for complete recovery or for a new baseline function to be established.
AKI is associated with increased risks of adverse outcomes such as progression to chronic kidney disease (CKD), end-stage renal disease (ESRD), and mortality. Therefore, early diagnosis, treatment, and proper follow-up are essential.
DiagnosisTop
1. Blood laboratory tests:
1) Serum creatinine and urea/blood urea nitrogen (BUN): Increased levels of serum creatinine and urea are important in assessing renal injury. However, a comparison of the currently increased serum creatinine and urea levels with previous levels aids in determining the duration and severity of kidney injury. In intrinsic AKI the daily serum creatinine increments are 44 to 88 micromol/L (0.5-1 mg/dL). In conditions associated with tissue catabolism, such as sepsis or crush syndrome, daily serum creatinine increments are significantly higher (as high as >176 micromol/L [2 mg/dL]) and are usually accompanied by severe acidosis and significant hyperkalemia. Estimation of GFR using the Cockcroft–Gault or Modification of Diet in Renal Disease (MDRD) formulas (see Chronic Kidney Disease) is not useful in describing or monitoring AKI.
2) Potassium: Hyperkalemia usually occurs in patients with reduced urine volumes and can be life–threatening if levels are >6.5 mmol/L. It is necessary to assess potassium concentrations in the context of the associated acid–base balance, because acidosis causes shifts of K+ from the intracellular compartment to the extracellular compartment. Also see Hyperkalemia.
3) Hypocalcemia and hyperphosphatemia may be significant in patients with crush syndrome and in those with tumor lysis syndrome (TLS), whereas hypercalcemia is usually seen in patients with a malignancy (eg, myeloma). Also see Hypercalcemia.
4) Uric acid: Hyperuricemia may be a sign of gout or TLS.
5) Creatine kinase (CK) and myoglobin levels: Increased CK and myoglobin levels are seen in patients with crush syndrome or rhabdomyolysis (eg, caused by statins, trauma, hyperthermia, electrical shock, and severe viral and bacterial infections).
6) Arterial blood gases: Metabolic acidosis may be associated with AKI. If severe metabolic acidosis is observed, then the plasma anion gap (PAG) and osmolar gap (OG) should be calculated to determine if AKI resulted from the ingestion of toxic substances (with methanol and ethylene glycol leading to most profound metabolic acidosis).
7) Anemia: Often a common feature of CKD. In AKI it may develop due to hemolysis or blood loss. Schistocytes are often seen on peripheral smear in the case of HUS or TTP.
8) Thrombocytopenia: Associated with HUS, TTP, and DIC.
2. Urine evaluation (Table 2, Table 3): This is a particularly important noninvasive test in the initial evaluation of AKI. The following parameters should be assessed: urine output, urinalysis, urine sediment, urine electrolytes, and urine osmolality. This will aid in differential diagnosis and guide further workup.
3. Electrocardiography (ECG): This should be performed particularly if there are significant electrolyte disturbances, as cardiac activity may be affected.
4. Imaging studies: Routine studies include ultrasonography and radiography. Renal ultrasonography can be used to exclude obstruction and evaluate kidney size and parenchyma. However, false-negative results of obstruction can occur in patients with severe volume depletion or with retroperitoneal fibrosis. Chest radiographs are helpful in evaluating pulmonary congestion and infiltrates.
5. Kidney biopsy: Biopsy should be obtained in situations when prerenal and postrenal causes of AKI have been excluded and the cause of intrinsic renal injury is unclear. Specifically, biopsy should be performed in patients in whom the probable or possible diagnosis will alter treatment, such as patients with oliguria who have rapidly worsening AKI with hematuria and high suspicion for GN, systemic vasculitis, acute interstitial nephritis, or who need immunosuppressive medications.
As indicated, AKI pertains to acute injury (within 7 days). The term acute kidney disease (AKD) is usually used to denote renal dysfunction that occurred within the last 7 days to 3 months, and the term chronic kidney disease, to denote nonrecovery of AKI/AKD after 3 months.
AKI is diagnosed if either of the following criteria is met:
1) A rapid increase in serum creatinine concentrations, that is, by ≥26.5 micromol/L (0.3 mg/dL) within 48 hours or a ≥1.5-fold increase in serum creatinine within the prior 7 days.
2) Urine volume <0.5 mL/kg/h for 6 hours.
Identification of causes of AKI is based on a thorough medical history, physical examination, and results of laboratory tests.
It is important to differentiate prerenal and postrenal AKI from intrinsic AKI, as rapid improvement of renal perfusion or release of obstruction leads to improved renal function. Markers may be useful for distinguishing prerenal and intrinsic causes and for differential diagnosis (Table 4). In patients with AKI superimposed on a preexisting CKD, the causes and differential diagnosis can vary (Table 5). Postrenal AKI is confirmed by ultrasonographic evidence of urinary flow obstruction in the renal pelvis, ureters, or bladder.
TreatmentTop
1. All efforts should be made to eliminate the causes of AKI and to avoid factors that could worsen renal function, particularly nephrotoxic drugs.
2. Monitoring of fluid balance, including urine output, fluid intake, and daily body weight.
3. Frequent monitoring (at least daily) of serum creatinine, urea, potassium, sodium, and bicarbonate. Complete blood count (CBC), blood gases, calcium, phosphate, and uric acid should be checked when deemed necessary. If severe metabolic acidosis is observed, the PAG and OG should be calculated.
4. Adjust drug dosage to the severity of renal failure (note that GFR assessment in patients with AKI may not be reliable).
5. Provide appropriate nutrition: We recommend that protein intake should not be restricted to prevent or delay starting renal replacement therapy (RRT) in AKI as malnutrition has been associated with an increased likelihood of death, complications, and use of health-care resources.Evidence 1Weak recommendation (downsides likely outweigh benefits, but the balance is close or uncertain; an alternative course of action may be better for some patients). Low Quality of Evidence (low confidence that we know true effects of the intervention). Quality of Evidence lowered due to the observational nature of data, indirectness, and imprecision. Fiaccadori E, Lombardi M, Leonardi S, Rotelli CF, Tortorella G, Borghetti A. Prevalence and clinical outcome associated with preexisting malnutrition in acute renal failure: a prospective cohort study. J Am Soc Nephrol. 1999 Mar;10(3):581-93. PubMed PMID: 10073609. Dietary protein or amino acids should be 0.8 to 1 g/kg/d in AKI patients without significant hypercatabolism not requiring RRT or 1 to 1.5 g/kg/d (maximum, 1.7 g/kg/d) in those with hypercatabolism or undergoing RRT. Carbohydrates, which are the principal source of energy, should be up to 5 g of glucose per kg a day. Fat should be between 0.8 to 1.2 g/kg/d. The maximum energy intake should be 35 kcal/kg/d. Standard commercially available diets are appropriate for the majority of patients with AKI who do not have significant hypercatabolism.Evidence 2Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). Low Quality of Evidence (low confidence that we know true effects of the intervention). Quality of Evidence lowered due to the observational nature of data, indirectness, and imprecision. Fiaccadori E, Maggiore U, Rotelli C, et al. Effects of different energy intakes on nitrogen balance in patients with acute renal failure: a pilot study. Nephrol Dial Transplant. 2005 Sep;20(9):1976-80. Epub 2005 Jul 5. PubMed PMID: 15998652.
Treatment of Underlying Conditions
In some patients further kidney injury can be prevented by appropriate treatment of underlying conditions.
1. Prerenal AKI: Treatment is aimed at addressing the underlying causes: shock and heart failure. In patients without hemorrhagic shock, volume restoration with crystalloids or albumin (rather than synthetic colloids) is recommended for initial management to restore effective circulatory volume.Evidence 3Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of the intervention). Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R; SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004 May 27;350(22):2247-56. PubMed PMID: 15163774. Myburgh JA, Finfer S, Bellomo R, et al; CHEST Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med. 2012 Nov 15;367(20):1901-11. doi: 10.1056/NEJMoa1209759. Epub 2012 Oct 17. Erratum in: N Engl J Med. 2016 Mar 31;374(13):1298. PubMed PMID: 23075127. Rochwerg B, Alhazzani W, Sindi A, et al; Fluids in Sepsis and Septic Shock Group. Fluid resuscitation in sepsis: a systematic review and network meta-analysis. Ann Intern Med. 2014 Sep 2;161(5):347-55. doi: 10.7326/M14-0178. Review. PubMed PMID: 25047428. Normal renal perfusion can prevent the transition of prerenal azotemia to intrinsic AKI and may lead to normalization of renal function within 1 to 3 days. Albumin can be considered to aid in reaching resuscitation goals, avoid excessive fluid administration in patients requiring large-volume resuscitation, or in specific patient subgroups (eg, a patient with cirrhosis and spontaneous peritonitis, patients with burns). Withdrawal of nephrotoxic agents such as diuretics, NSAIDs, ACEIs, and ARBs is important to avoid further damage.
2. Intrinsic AKI: Treatment is aimed at addressing the underlying cause of kidney disease. Supportive care is necessary to eliminate any life-threatening complications, such as hypotension or severe metabolic complications.
3. Postrenal AKI: Treatment is aimed at rapid resolution of the urinary tract obstruction. In the postobstructive phase, specific attention should be paid to urine output, volume status, and electrolytes. Usually patients with polyuria should receive in the beginning 0.45% saline at a replacement rate of approximately two-thirds of the urinary losses. The replacement fluid rate can be decreased over several days and the concentration of administered saline can be adjusted depending on the results of serum biochemical testing.
The most commonly used types of RRT are intermittent or daily hemodialysis (HD), continuous renal replacement therapy (CRRT), sustained low-efficiency dialysis (SLED), and peritoneal dialysis (PD). The decision on when to start RRT should be based on clinical and biochemical features and involves individual clinical judgement. The most recent evidence shows that early RRT initiation in critically ill adults does not lead to improved outcomes, particularly mortality.Evidence 4Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due to the risk of bias, heterogeneity, and imprecision. Karvellas CJ, Farhat MR, Sajjad I, et al. A comparison of early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury: a systematic review and meta-analysis. Crit Care. 2011;15(1):R72. doi: 10.1186/cc10061. Epub 2011 Feb 25. Review. PubMed PMID: 21352532; PubMed Central PMCID: PMC3222005. Gaudry S, Hajage D, Schortgen F, et al; AKIKI Study Group. Initiation Strategies for Renal-Replacement Therapy in the Intensive Care Unit. N Engl J Med. 2016 Jul 14;375(2):122-33. doi: 10.1056/NEJMoa1603017. Epub 2016 May 15. PubMed PMID: 27181456. Bhatt GC, Das RR. Early versus late initiation of renal replacement therapy in patients with acute kidney injury-a systematic review & meta-analysis of randomized controlled trials. BMC Nephrol. 2017 Feb 28;18(1):78. doi: 10.1186/s12882-017-0486-9. PubMed PMID: 28245793; PubMed Central PMCID: PMC5331682. STARRT-AKI Investigators; Canadian Critical Care Trials Group; Australian and New Zealand Intensive Care Society Clinical Trials Group; United Kingdom Critical Care Research Group; Canadian Nephrology Trials Network; Irish Critical Care Trials Group; Bagshaw SM, Wald R, Adhikari NKJ, et al. Timing of Initiation of Renal-Replacement Therapy in Acute Kidney Injury. N Engl J Med. 2020 Jul 16;383(3):240-251. doi: 10.1056/NEJMoa2000741. PMID: 32668114.
1. Clinical indications: Volume overload (pulmonary edema) refractory to diuretic agents, uremic encephalopathy (altered mental status or seizures), pericarditis, uremic coagulopathy, overdosing certain drugs, and substance poisoning.
2. Biochemical indications (approximations, ie, there is no decisive difference between a pH of 7.21 and 7.19): Refractory hyperkalemia (serum potassium >6.5 mmol/L), refractory metabolic acidosis (pH <7.2; bicarbonate level [HCO3−] <8 mmol/L), refractory hypercalcemia (>4.5 mmol/L), uremia 20 to 40 mmol/L (based on observational trials), refractory hypermagnesemia (3-5 mmol/L), severe dysnatremia (eg, <116 or >160 mmol/L; levels are reasonable examples only), or severe hyperuricemia in TLS.Evidence 5Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). Low Quality of Evidence (low confidence that we know true effects of the intervention). Quality of Evidence lowered due to the risk of bias, heterogeneity, and indirectness. Karvellas CJ, Farhat MR, Sajjad I, et al. A comparison of early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury: a systematic review and meta-analysis. Crit Care. 2011;15(1):R72. doi: 10.1186/cc10061. Epub 2011 Feb 25. Review. PubMed PMID: 21352532; PubMed Central PMCID: PMC3222005. Gaudry S, Hajage D, Schortgen F, et al; AKIKI Study Group. Initiation Strategies for Renal-Replacement Therapy in the Intensive Care Unit. N Engl J Med. 2016 Jul 14;375(2):122-33. doi: 10.1056/NEJMoa1603017. Epub 2016 May 15. PubMed PMID: 27181456. Bhatt GC, Das RR. Early versus late initiation of renal replacement therapy in patients with acute kidney injury-a systematic review & meta-analysis of randomized controlled trials. BMC Nephrol. 2017 Feb 28;18(1):78. doi: 10.1186/s12882-017-0486-9. PubMed PMID: 28245793; PubMed Central PMCID: PMC5331682.
3. Modality: Numerous studies and meta-analyses failed to show benefits of CRRT as compared with standard intermittent RRT.Evidence 6Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due to the risk of bias, heterogeneity, and imprecision. Schneider AG, Bellomo R, Bagshaw SM, et al. Choice of renal replacement therapy modality and dialysis dependence after acute kidney injury: a systematic review and meta-analysis. Intensive Care Med. 2013 Jun;39(6):987-97. doi: 10.1007/s00134-013-2864-5. Epub 2013 Feb 27. Review. PubMed PMID: 23443311. More studies are ongoing. Currently CRRT can be considered in patients with hemodynamic instability, acute brain injury, and increased intracranial pressure or generalized brain edema.
1. Volume overload: Restrict salt and water intake and administer a loop diuretic, eg, IV furosemide 40 mg. If this is ineffective, use additional doses up to a maximum of 500 mg/d of furosemide. Note that high doses of loop diuretics may cause hearing loss. Addition of a thiazide diuretic can be considered, such as oral metolazone 5 to 10 mg bid, to augment diuresis. The practice pattern on when to start RRT to remove excess water is variable.
2. Hyperkalemia: see Hyperkalemia.
3. Metabolic acidosis: Sodium bicarbonate (NaHCO3) is recommended in patients with severe acidosis to maintain a serum [HCO3−] >8 mmol/L or arterial pH >7.2.Evidence 7Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due to the risk of bias. Jaber S, Paugam C, Futier E, et al; BICAR-ICU Study Group. Sodium bicarbonate therapy for patients with severe metabolic acidaemia in the intensive care unit (BICAR-ICU): a multicentre, open-label, randomised controlled, phase 3 trial. Lancet. 2018 Jul 7;392(10141):31-40. doi: 10.1016/S0140-6736(18)31080-8. Epub 2018 Jun 14. Erratum in: Lancet. 2018 Dec 8;392(10163):2440. PubMed PMID: 29910040. Possible adverse effects are hypocalcemia and hypokalemia.
4. Hyperphosphatemia: see Hyperphosphatemia.
5. Anemia: Transfusion of packed red blood cells should be guided by the patient’s symptoms and clinical situation. Erythropoietic agents are not indicated in the treatment of AKI.
6. Coagulopathy leading to bleeding:
1) Desmopressin 0.3 microg/kg in an IV or subcutaneous infusion over 15 to 30 minutes or 3 microg/kg intranasally; the dose may be repeated after 6 hours. The effect of desmopressin on improving platelet function is short lasting, with bleeding times returning to baseline within 24 hours. Adverse effects include tachyphylaxis after one dose, headache, facial flushing, and rare thrombotic events.
2) Cryoprecipitate is a source of factor VIII, factor XIII, multimeric form of von Willebrand factor, fibrinogen, and fibronectin. Use 10 units IV over 30 minutes every 12 to 24 hours. Adverse effects include risk of transfusion-related infection, fever, and allergic reaction. Rare but severe reactions include anaphylaxis, pulmonary edema, and intravascular hemolysis.
3) Esterified estrogens 0.6 mg/kg IV over 30 to 40 minutes once daily for 5 days. The duration of action is up to 2 weeks. Esterified estrogens can be administered in men and if given for short periods have been associated with hot flashes only.
PrognosisTop
The overall mortality in patients with AKI is high, and AKI is considered an independent risk factor for mortality. Mortality rates are population-dependent, ranging between 10% and 80%. Patients with uncomplicated AKI have mortality rates of ~10%. Patients presenting with AKI and multiorgan failure have mortality rates >50%. In patients requiring RRT mortality rises to >80%. Death is usually a result of the severity of the underlying disease causing AKI rather than the renal injury itself. It has been shown that from 20% to 50% of patients with AKI progress to CKD and 3% to 15% develop ESRD, even those who initially recover sufficient kidney function to discontinue dialysis.
PreventionTop
1. Patients at increased risk of AKI should be identified and appropriate preventative measures should be undertaken as early as possible to treat the underlying diseases.
2. Institute early and intensive treatment of conditions causing reduction of effective circulating blood volume with appropriate IV fluids and close monitoring of volume and patient response.
3. Monitor urine output and periodically evaluate renal function in patients at increased risk of AKI.
4. Avoid nephrotoxic drugs, particularly in patients with impaired renal function.
5. Identify patients at increased risk for contrast nephropathy (see Special Considerations, below).
6. Patients at risk of AKI caused by myoglobinuria should be identified and hydration with 0.9% sodium chloride or sodium bicarbonate should be initiated.
Special ConsiderationsTop
1. Contrast-induced AKI (CI-AKI), previously called contrast-induced nephropathy (CIN), is defined as an impairment of renal function and measured as either a 25% increase in serum creatinine levels from baseline or an increase of 44 micromol/L (0.5 mg/dL) in the absolute serum creatinine value that develops within 1 to 3 days of administration of radiographic contrast agents. Its treatment, prevention, and definition itself remain controversial.
The exact pathophysiology of CI-AKI is unclear and appears to be multifactorial. Several theories suggest intrarenal vasoconstriction leading to medullary hypoxia, which is mediated by decreased production of nitric oxide and prostaglandin and increased levels of endothelin and adenosine. In addition, there is increased viscosity within the medullary vascular bed, which may further contribute to medullary hypoxia and renal injury. The second mechanism of injury is the generation of reactive oxygen species, leading to cytotoxic effects on the renal parenchyma. Lastly, the contrast media itself may be cytotoxic, leading to direct damage.
Patient risk factors for the development of CI-AKI include CKD, diabetes mellitus, intravascular volume depletion, decreased cardiac output, and concomitant use of nephrotoxic drugs. Other factors include high doses of radiocontrast, multiple procedures within 72 hours, intra-arterial administration, and high-osmolar contrast media.
Diagnosis is based on an early increase of serum creatinine levels of administration of a contrast agent (within 1-3 days) and exclusion of other potential causes, such as prerenal azotemia, AIN, cholesterol crystal embolization, and renal artery thromboembolism. Cholesterol crystal embolization can be distinguished from CI-AKI by abnormal renal function occurring at ~4 weeks after contrast exposure. Patients may have fever, headaches, intestinal ischemia, myalgia, livedo reticularis, and “blue toe syndrome.”
Prevention is the mainstay of therapy for CI-AKI and the following strategies should be considered:
1) Nonpharmacologic prevention strategies:
a) Identification of high-risk individuals and screening for both acute kidney disease and CKD is highly recommended.
b) Administer the lowest possible doses of contrast agents. Dosing per kilogram of body weight is recommended to reduce the amount of contrast media, particularly in patients with a low body mass index.Evidence 8Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). Low Quality of Evidence (low confidence that we know true effects of the intervention). Quality of Evidence lowered due to the observational nature of data. Cigarroa RG, Lange RA, Williams RH, Hillis LD. Dosing of contrast material to prevent contrast nephropathy in patients with renal disease. Am J Med. 1989 Jun;86(6 Pt 1):649-52. PubMed PMID: 2729314.
c) Use of either iso-osmolar or low-osmolar iodinated contrast media is recommended over high-osmolar iodinated contrast media.Evidence 9Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due to the risk of bias and heterogeneity. Barrett BJ, Carlisle EJ. Metaanalysis of the relative nephrotoxicity of high- and low-osmolality iodinated contrast media. Radiology. 1993 Jul;188(1):171-8. PubMed PMID: 8511292. Heinrich MC, Häberle L, Müller V, Bautz W, Uder M. Nephrotoxicity of iso-osmolar iodixanol compared with nonionic low-osmolar contrast media: meta-analysis of randomized controlled trials. Radiology. 2009 Jan;250(1):68-86. doi: 10.1148/radiol.2501080833. PubMed PMID: 19092091.
d) Avoidance of concomitant use of nephrotoxic drugs.
2) Pharmacologic prevention strategies: Note that this is a subject of ongoing debate, with meta-analyses generally showing low quality of evidenceEvidence 10Low Quality of Evidence (low confidence that we know true effects of the intervention). Quality of Evidence lowered due to the risk of bias, heterogeneity, and indirectness. Subramaniam RM, Suarez-Cuervo C, Wilson RF, et al. Effectiveness of Prevention Strategies for Contrast-Induced Nephropathy: A Systematic Review and Meta-analysis. Ann Intern Med. 2016 Mar 15;164(6):406-16. doi: 10.7326/M15-1456. Epub 2016 Feb 2. Review. PubMed PMID: 26830221. and more recent randomized controlled trials not showing benefit of bicarbonates or N-acetylcysteine.Evidence 11Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due to indirectness. Weisbord SD, Gallagher M, Jneid H, et al; PRESERVE Trial Group. Outcomes after Angiography with Sodium Bicarbonate and Acetylcysteine. N Engl J Med. 2018 Feb 15;378(7):603-614. doi: 10.1056/NEJMoa1710933. Epub 2017 Nov 12. PubMed PMID: 29130810. Among patients at high risk of developing CI-AKI consideration is given to the use of:
a) Volume resuscitation with the use of IV hydration with either 0.9% saline or NaHCO3 (154 mmol/L in 5% glucose [dextrose]) 1 to 1.5 mL/kg/h for 3 to 6 hours before and 6 to 12 hours after the administration of a contrast agent.
b) N-acetylcysteine 1200 mg orally or IV bid for 48 hours, beginning 1 day prior to the procedure.
c) Prophylactic intermittent HD or hemofiltration for contrast media removal is not recommended for the prevention of AKI as it may increase its risk.Evidence 12Strong recommendation (downsides clearly outweigh benefits; right action for all or almost all patients). Low Quality of Evidence (low confidence that we know true effects of the intervention). Quality of Evidence lowered due to the risk of bias and heterogeneity. Cruz DN, Goh CY, Marenzi G, Corradi V, Ronco C, Perazella MA. Renal replacement therapies for prevention of radiocontrast-induced nephropathy: a systematic review. Am J Med. 2012 Jan;125(1):66-78.e3. doi: 10.1016/j.amjmed.2011.06.029. Review. PubMed PMID: 22195531.
2. Acute phosphate nephropathy is a form of AKI that is caused by rapidly developing nephrocalcinosis following oral administration of phosphate-containing agents (usually sodium phosphate) used for bowel preparation before colonoscopy. Renal failure is usually seen within a few days of phosphate administration. Some patients may have a subacute presentation and develop AKI over weeks to months after ingestion of these preparations. Acute phosphate nephropathy is frequently preceded by symptoms of acute hyperphosphatemia and hypocalcemia, including tetany, altered mental status, and hypotension. Renal deposition of calcium phosphate precipitants along with tubulointerstitial inflammation is seen on biopsy and is irreversible.
Risk factors associated with the development of acute phosphate nephropathy include advanced age, preexisting CKD, dehydration, use of renin-angiotensin-aldosterone system (RAAS) blockade, NSAIDs, diuretics, and high-dose phosphates.
Prevention: The most important prevention strategy is identification of high-risk patients and avoidance of phosphate agents in this group. Patients at increased risk are elderly, with hypertension, and have low estimated GFR (<60 mL/min/1.73 m2). Rigorous attention should be paid to adequate hydration and avoidance of RAAS blockade, diuretics, and NSAIDs on the day before and day of the procedure.
Treatment: There is no specific treatment for acute phosphate nephropathy. Patients should be treated with supportive care.
3. Anticoagulant-related nephropathy (ARN): Also referred to as warfarin-related nephropathy; this is a poorly understood and fairly underreported cause of AKI associated with glomerular hemorrhage and renal tubular obstruction by red blood cell casts. Clinically it is characterized by a sudden and unexplained irreversible deterioration of renal function without hematuria in patients treated with warfarin or other anticoagulants. A major risk factor for ARN is moderate or severe coagulopathy induced by warfarin or other anticoagulants.
The incidence of ARN is difficult to determine, but the disease may develop in up to 20% of patients with excessive anticoagulation, most frequently within 1 year after initiation of therapy. The majority of reported cases involve patients with preexisting CKD, particularly with nephrotic syndrome. Additional risk factors include advanced age, diabetes mellitus, hypertension, and cardiovascular disease. At this time, little is known about the exact pathophysiology or therapeutic approach. Minimizing exposure to the anticoagulant and avoiding excessive INR is recommended, especially in patients with CKD, to reduce the risk of this condition.
4. Abdominal compartment syndrome (ACS) refers to organ dysfunction caused by increased intra-abdominal pressure leading to impairment of blood supply to various organs, including kidneys. This condition is likely underdiagnosed among critically ill patients.
The causes of ACS can be categorized as primary (sometimes requiring surgery), secondary (medical), and recurrent (Table 6). Primary conditions are disorders that may require surgical intervention. Secondary conditions are usually managed medically. Recurrent conditions are disorders in which ACS redevelops after either medical or surgical treatment. ACS should be suspected in patients who develop oliguria in the course of intestinal obstruction and respiratory failure. Diagnosis is confirmed by sustained intra-abdominal pressure >20 mm Hg (with or without abdominal perfusion pressure <60 mm Hg) associated with new organ dysfunction or failure. Once the diagnosis of intra-abdominal hypertension (IAH) has been established, the goal is to decrease the intra-abdominal pressure to <15 mm Hg.
5. Hepatorenal syndrome: see Cirrhosis.
6. Acute renal cortical necrosis (ARCN) is a very rare cause of AKI in resource-abundant countries. It is caused by diminished renal arterial perfusion that leads to cortical ischemia. It is prevalent in pregnant women, usually in advanced pregnancy, as a result of accidental hemorrhage or placental abruption. Rare causes include complications of intrauterine fetal death, sepsis, preeclampsia, or amniotic fluid embolism. The precipitating event is probably intravascular coagulation or severe renal ischemia.
ARCN presents with sudden decrease of urine volume or anuria, which is frequently accompanied by gross hematuria, lumbar pain, and hypotension. The presence of the triad of symptoms, including anuria, gross hematuria, and lumbar pain, differentiates ARCN from other forms of AKI in pregnancy.
Diagnosis is suspected on the basis of the triad of symptoms. The diagnosis can usually be established by ultrasonography or CT. CT angiography is preferred despite the risk of contrast nephropathy. Alternatively isotopic penta-acetic acid scanning can be used. In the acute phase imaging studies show patchy or diffuse densities in the renal cortex, which appear as hypoechogenic areas on ultrasonography and hypodensity on CT scans. After 1 to 2 months calcifications in the renal cortex may be seen on plain abdominal radiographs. Renal biopsy is rarely needed and performed only if the diagnosis is unclear.
Prognosis is poor, with mortality rates at 1 year >20% and partial renal recovery rates ≤40%.
Treatment is aimed at addressing the underlying condition. Patients should be treated with supportive management.
Also see Tumor Lysis Syndrome.
Tables and FiguresTop
|
Stage |
Serum creatinine |
Urine output |
|
1 |
Increase 1.5-1.9 × baseline or Increase by ≥26.5 micromol/L (≥0.3 mg/dL) |
<0.5 mL/kg/h for 6-12 h |
|
2 |
2-2.9 × baseline |
<0.5 mL/kg/h for ≥12 h |
|
3 |
3 × baseline or Serum creatinine ≥353.6 micromol/L (≥4.0 mg/d) or Initiation of RRT |
<0.3 mL/kg/h for ≥24 h or Anuria for ≥12 h |
|
Based on Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Inter., Suppl. 2012; 2: 1-138. |
||
|
KDIGO, Kidney Disease: Improving Global Outcomes; RRT, renal replacement therapy. |
||
|
Component |
Interpretation |
|
Specific gravity |
Estimates urine osmolality – Values <1.010: Hydrated state – Values <1.005: Diabetes insipidus or water intoxication – Values >1.035: Dehydration, high glucose levels, IV contrast |
|
pH |
Normal range: 4.5-8.0 – High urine pH: Distal RTA, infections with urea-splitting organisms, vegetarians – Low urine pH: Metabolic acidosis, dehydration, high-protein diet |
|
RBCs |
Positive result may indicate hematuria, hemoglobinuria, or myoglobinuria |
|
WBCs |
Suggest inflammation: UTI, GN, or AIN |
|
Ketones |
Not normally found in urine. Dipstick tests for presence of acetoacetic acid but not acetone or beta-hydroxybutyric acid. Positive results are associated with uncontrolled diabetes, pregnancy with diabetes, carbohydrate-free diets, and starvation |
|
Glucose |
Nearly all glucose filtered by glomeruli is reabsorbed in proximal tubules and undetectable in healthy patients. Positive results are associated with hyperglycemia, pregnancy, and Fanconi syndrome |
|
Bilirubin |
Unconjugated bilirubin is not present in urine. Conjugated bilirubin appears in liver disease or obstruction of bile ducts |
|
AIN, acute interstitial nephritis; GN, glomerulonephritis; RBC, red blood cell; RTA, renal tubular acidosis; UTI, urinary tract infection; WBC, white blood cell. | |
|
Features |
Comments |
|
Cells |
1) >3 RBCs/HPF: Quantity and morphology assessment required; dysmorphic RBCs are associated with GN 2) WBCs: Associated with infection or inflammation: a) PMNs associated with infection b) Eosinophilia associated with AIN, cholesterol emboli, or schistosomiasis 3) Epithelial cells: a) Squamous cells are usually contaminants b) Transitional cells usually due to bladder irrigation or catheterization, rarely due to malignancy c) Renal tubular cells seen in ATN |
|
Casts |
1) Hyaline cast: Nonspecific, seen in strenuous exercise and dehydration 2) Granular cast (muddy-brown cast): Seen in ATN 3) Waxy cast: Seen in CKD 4) Fatty cast: Seen in nephrotic syndrome, mercury and ethylene glycol poisoning 5) RBC cast: Seen in GN 6) WBC cast: Seen in AIN, pyelonephritis, and GN |
|
Crystals |
1) Acidic urine: Uric acid or calcium oxalate 2) Alkaline urine: Triple phosphate or calcium phosphate |
|
AIN, acute interstitial nephritis; ATN, acute tubular necrosis; GN, glomerulonephritis; HPF, high-power field; RBC, red blood cell; PMN, polymorphonuclear neutrophil; WBC, white blood cell. | |
|
|
Prerenal AKI |
Intrinsic AKI |
|
Daily urine output (mL) |
<400 |
Variable |
|
Urine osmolality (mOsm/kg H2O) |
>500 |
<350 |
|
Urine specific gravity (g/mL) |
>1.023 |
≤1.012 |
|
Ratio of urea (mmol/L) to serum creatinine (micromol/L) |
>1:10 |
<1:10 |
|
Ratio of urinary to serum creatinine concentration |
>40 |
<20 |
|
Urinary [Na+] concentration (mmol/L)a |
<20 |
>40 |
|
Fractional excretion of sodiumb |
<1% |
>2% |
|
Urine sediment |
No abnormal findings or hyaline casts only |
Epithelial cells, pigmented muddy-brown granular casts or tubular epithelial cell casts |
|
a Urinary sodium concentration (this should be measured before administering furosemide). b Fractional excretion of sodium: FENa = [(urinary Na concentration in mmol/L × serum creatinine concentration in mg/dL) / (serum Na concentration in mmol/L × urinary creatinine concentration in mg/dL)] × 100%. | ||
|
AKI, acute kidney injury. | ||
|
|
AKI |
CKD |
|
History suggestive of CKD |
No |
Yes |
|
Kidney size |
Normal |
Small |
|
Dynamics of serum creatinine increases |
High |
Low |
|
CBC |
Normal |
Anemia |
|
Calcium‑phosphate metabolism |
Mild or moderate disturbances (depending on AKI etiology) |
High phosphate level, increased alkaline phosphatase level, radiographic signs of renal osteodystrophy and/or soft tissue calcifications |
|
Fundoscopy |
Usually normal |
Frequently lesions typical of diabetes mellitus or chronic hypertension |
|
AKI, acute kidney injury; CBC, complete blood count; CKD, chronic kidney disease. | ||
|
Primary |
– Massive intra-abdominal, retroperitoneal, or pelvic bleeding – Liver transplant – Mechanical intestinal obstruction – Postoperative closure of abdomen under tension |
|
Secondary |
– Large-volume fluid replacement – Pancreatitis – Severe intra-abdominal infection – Ascites – Ileus – Major burns – Continuous ambulatory peritoneal dialysis |
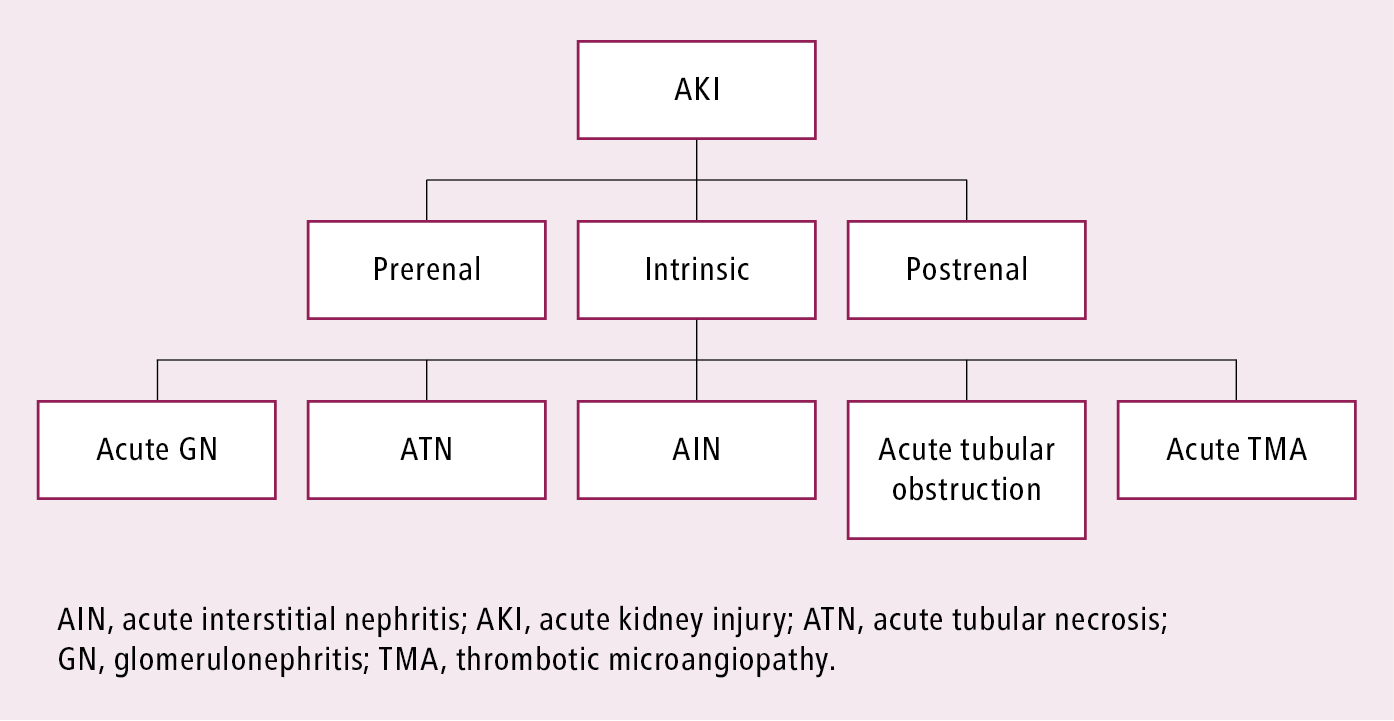
Figure 11.1-1. Basic classification of causes of acute kidney injury.