Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020 Jun;79(6):685-699. doi: 10.1136/annrheumdis-2019-216655. Epub 2020 Jan 22. PMID: 31969328.
Solomon DH, Bitton A, Katz JN, Radner H, Brown EM, Fraenkel L. Review: treat to target in rheumatoid arthritis: fact, fiction, or hypothesis? Arthritis Rheumatol. 2014 Apr;66(4):775-82. doi: 10.1002/art.38323. Review. PubMed PMID: 24757129; PubMed Central PMCID: PMC4012860.
Smolen JS, Landewé R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014 Mar;73(3):492-509. doi: 10.1136/annrheumdis-2013-204573. Epub 2013 Oct 25. PubMed PMID: 24161836; PubMed Central PMCID: PMC3933074.
Colebatch AN, Edwards CJ, Østergaard M, et al. EULAR recommendations for the use of imaging of the joints in the clinical management of rheumatoid arthritis. Ann Rheum Dis. 2013 Jun;72(6):804-14. doi: 10.1136/annrheumdis-2012-203158. Epub 2013 Mar 21. PubMed PMID: 23520036.
van der Heijde D, van der Helm-van Mil AH, Aletaha D, et al. EULAR definition of erosive disease in light of the 2010 ACR/EULAR rheumatoid arthritis classification criteria. Ann Rheum Dis. 2013 Apr;72(4):479-81. doi: 10.1136/annrheumdis-2012-202779. Epub 2013 Feb 2. PubMed PMID: 23378540.
Singh JA, Furst DE, Bharat A, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012 May;64(5):625-39. doi: 10.1002/acr.21641. Review. PubMed PMID: 22473917; PubMed Central PMCID: PMC4081542.
Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010 Sep;69(9):1580-8. doi: 10.1136/ard.2010.138461. Erratum in: Ann Rheum Dis. 2010 Oct;69(10):1892. PubMed PMID: 20699241.
Chakravarty K, McDonald H, Pullar T, et al; British Society for Rheumatology, British Health Professionals in Rheumatology Standards, Guidelines and Audit Working Group; British Association of Dermatologists (BAD). BSR/BHPR guideline for disease-modifying anti-rheumatic drug (DMARD) therapy in consultation with the British Association of Dermatologists. Rheumatology (Oxford). 2008 Jun;47(6):924-5. Epub 2006 Aug 28. PubMed PMID: 16940305.
Definition, Etiology, PathogenesisTop
Rheumatoid arthritis (RA) is a chronic systemic autoimmune connective tissue disease of unknown etiology characterized by symmetric polyarthritis and associated extra-articular and systemic manifestations. RA leads to impairment, disability, and premature death if treated inadequately. The onset and progression of the disease is considered to be related to the response of CD45RO+ T cells (memory cells) to an unknown antigen or antigens, exogenous or endogenous, in a genetically predisposed individual. Based on the presence or absence of autoantibodies (IgM rheumatoid factor [RF] and/or anti-citrullinated protein antibodies [ACPAs; also termed antibodies to cyclic citrullinated peptides]), the disease can be divided into seropositive RA and seronegative RA, respectively.
Clinical Features and Natural HistoryTop
RA affects women 3 times as often as men, with the peak age of onset occurring between 30 and 50 years. In ~70% of patients with RA, relapses and partial remissions are observed, leading to progressive joint destruction. In ~15% of patients the course of RA is mild, with moderate disease activity, involvement of a few joints, and slowly progressive joint destruction. In ~10% of patients remissions are prolonged, lasting up to several years. Very rarely, the course of the disease is episodic (so-called palindromic rheumatism) or self-limiting. Up to 25% of these seropositive patients will go on to develop RA in subsequent years. Usually the disease onset is insidious, over a few weeks to months; however, in 10% to 15% of patients signs and symptoms develop rapidly over a few days (in these cases joint involvement may be asymmetric). In 75% of pregnant patients, symptoms improve in the first trimester and worsen after delivery. RA itself does not increase the risk of maternal or fetal complications.
1. Typical signs and symptoms: Symmetric pain and swelling of the joints of hands and feet, less frequently also of larger joints (eg, knee or shoulder). Morning joint stiffness of variable duration, usually lasting >1 hour.
2. Systemic manifestations: Low-grade fever, myalgia, fatigue, anorexia, and weight loss can occur. These usually precede the diagnosis.
3. Musculoskeletal manifestations: Early RA usually presents with a symmetric polyarthritis particularly affecting wrists, hands (metacarpophalangeal [MCP] and proximal interphalangeal [PIP] joints), and feet (metatarsophalangeal [MTP] joints), although the disease can present with large joint polyarthritis. The lumbar spine, distal interphalangeal (DIP) joints, and hip joints are unlikely to be involved in the inflammatory arthritis process. Joints of the upper extremities (particularly wrist joints) are affected more frequently than those of the lower extremities, although MTP joint involvement may be the first sign of RA. The onset of RA may be atypical, manifesting as monoarthritis or as palindromic rheumatism (pain and/or swelling in various joints lasting from a few hours to a few days and remitting spontaneously). Features occurring in the early stages of RA include heat (without erythema of the overlying skin), tenderness and swelling of joints and tendons, and joint effusions.
1) Joints of the hand (see Fingers in Rheumatic Diseases): In patients with early RA, fusiform swelling of PIP and MCP joints and palmar erythema located on the thenar and hypothenar eminences may be observed. Deformities develop in patients with more advanced RA. Atrophy of the interosseous and lumbrical muscles may occur due to disuse or neuropathy. Ulnar deviation of digits is the most frequent deformity. In later stages patients may develop volar subluxation of the phalanges at the level of the MCP joints, hyperextension of the PIP joint with flexion of the DIP joint (“swan-neck deformity”), or flexion of the PIP joint with hyperextension of the DIP joint (“boutonnière deformity,” caused by involvement of ligaments and tendons and muscle contractures), which lead to a significant impairment of finger movement. Wrist involvement includes dorsal or palmar swelling at the radiocarpal and carpal joints, swelling around the extensor carpi ulnaris tendon, and erosion of the ulnar styloid with interruption of the supporting ligaments, resulting in the “piano key” sign. The expanding synovium may compress the median nerve under the flexor retinaculum at the wrist, leading to carpal tunnel syndrome.
2) Elbow: Pain, swelling, and limited extension. Some patients may develop permanent flexion contractures.
3) Shoulder: Synovitis of the glenohumeral joint, subacromial-subdeltoid bursitis, rotator cuff tendonitis (causing subluxation), and atrophy of adjacent muscles. The acromioclavicular joint is rarely involved in RA (usually due to degenerative arthritis).
4) MTP joints are very frequently involved from the onset of the disease, causing toe deformities similar to those of the fingers.
5) Ankle and midfoot joints may be involved in progressive RA, often resulting in instability.
6) Hip involvement is uncommon in RA and is more commonly found in osteoarthritis. Hip synovitis presents with pain in the groin and progressive difficulty in walking.
7) Knees are frequently involved in patients with RA. Suprapatellar joint effusion results in a positive patellar tap test or swelling of the medial and/or lateral aspects of the joint, which increases upon compression of the suprapatellar region. A Baker cyst may develop (causing a palpable popliteal protrusion); increasing pressure of the accumulating effusion may lead to a rupture of the Baker cyst, leakage of the synovial fluid into tissues of the lower leg, significant lower leg edema, and worsening of knee pain with concomitant contracture of the knee (this needs to be differentiated from deep vein thrombosis of the lower leg). When this is accompanied by blood tracking down to the ankle, a crescent sign may be seen (discoloration around the lateral malleolus).
8) Spine: The cervical spine can be affected, leading to subluxation, destruction of the cartilage of the intervertebral disk, and disk prolapse. Atlantoaxial subluxation is potentially dangerous and presents with pain referred to the occipital area, paresthesias within the shoulder girdle and the upper extremity, and spastic paraplegia in the case of spinal cord compression.
9) Other joints: Involvement of the temporomandibular joint (pain in the temporomandibular area, difficulty opening the mouth and eating), cricoarytenoid joint (hoarseness), and less commonly the sternoclavicular joint.
4. Extra-articular manifestations occur particularly in patients with severe, long-standing seropositive RA and include:
1) Painless subcutaneous nodules on extensor surfaces, in particular of the forearm but also in pressure areas (eg, buttocks), within tendons, and over the joints. Nodules may also develop within internal organs.
2) Cardiovascular manifestations: Atherosclerosis and thromboembolic events (cardiovascular events are a common cause of death in patients with RA), pericarditis (in advanced RA), pericardial effusion that can be clinically asymptomatic, myocardial and valvular lesions (nodules, cardiomyopathy), pulmonary hypertension.
3) Pulmonary manifestations: Pleuritis (effusion is often clinically asymptomatic), nodules in the lungs (which may undergo fibrosis, calcification, or infection, and may be mistaken for neoplastic lesions), obliterative bronchiolitis, pulmonary fibrosis.
4) Ocular manifestations: Dry conjunctiva in the course of secondary Sjögren syndrome, episcleritis, and rarely scleritis (“corneal melt”).
5) Renal manifestations (mainly due to adverse effects of drugs used in RA treatment): Interstitial nephritis, pyelonephritis, secondary amyloidosis (complication of chronic active inflammation).
6) Other: Small- and medium-vessel vasculitis (this may lead to necrosis of distal digits, skin, and internal organs); involvement of the nervous system: carpal tunnel syndrome, polyneuropathy (particularly in the course of vasculitis), mononeuritis multiplex related to vasculitis, spinal nerve root compression due to destruction of cervical spine joints; submaxillary, cervical, axillary, and cubital lymphadenopathy; splenomegaly (with leukopenia [neutropenia] as part of Felty syndrome).
DiagnosisTop
1. Laboratory tests: Elevated erythrocyte sedimentation rate (ESR) (>30 mm/h) and/or elevated C-reactive protein (CRP) levels, anemia (may be normocytic normochromic in anemia of chronic disease, microcytic hypochromic in iron deficiency anemia secondary to gastrointestinal blood loss, or macrocytic related to folate deficiency [methotrexate—MTX—use] or B12 deficiency [pernicious anemia can be associated with RA]), minor elevations in the white blood cell count with a normal differential count or leukopenia associated with disease-modifying antirheumatic drug (DMARD) use, thrombocytosis (in patients with highly active disease) or thrombocytopenia (adverse effect of drugs), elevated plasma alpha1-globulin and alpha2-globulin levels, positive serum IgM RF in ~75% of patients (high titers correlate with rapid joint destruction and presence of extra-articular features), ACPAs (sensitivity >50% and specificity >98% in patients with RA; ~40% of patients with negative IgM RF have positive ACPAs, which are an adverse prognostic factor [similar to RF] and predict rapid joint destruction). A new test, 14-3-3eta, is used in conjunction with the other autoantibodies and confers greater specificity and sensitivity. A triple antibody test is available commercially as “JOINTstat” (RF, ACPA, 14-3-3eta).
2. Synovial fluid analysis: Features of inflammation. Other than when excluding infection, synovial fluid is not routinely examined in RA. If done, some clinicians consider testing for RF, which may be detected earlier than plasma RF. In some patients ragocytes may be observed (neutrophils, macrophages, monocytes, or synoviocytes with cytoplasmic inclusions of phagocytosed immune complexes).
3. Imaging studies: Radiographs of the affected joints reveal lesions that correlate with stage of the disease and may show 4 typical features: soft tissue swelling, joint space narrowing, periarticular osteopenia, and/or marginal erosions. Ultrasonography reveals synovial thickening, increased synovial vascularity, and effusions and erosions in small and large joints; joint erosions may be detected earlier by ultrasonography than by radiography. Magnetic resonance imaging (MRI) allows for early detection of synovitis, joint erosions, and bone marrow edema, which may precede synovitis. Computed tomography (CT) detects joint destruction earlier than radiographs and is the best method for visualizing bone cysts in patients with preserved or only slightly altered continuity of the bone cortex (in such cases the MRI signal of the cysts is normal and they may not be visualized); it is particularly useful in evaluating lesions in the cervical spine.
Basic laboratory investigations that should be undertaken include the ESR, CRP, IgM RF, ACPAs, complete blood count with a differential count, serum alanine aminotransferase (ALT), serum aspartate aminotransferase (AST), uric acid (if gout is suspected in the differential diagnosis), creatinine and electrolyte levels, urinalysis, synovial fluid analysis (to exclude other joint pathologies in patients with joint effusion [specifically infections or crystal arthropathies]), and radiographs of the hands, feet, or other affected joints. In patients with normal radiographs, ultrasonography with power Doppler and/or MRI could be performed to assess for early disease activity. Antinuclear antibody (ANA) levels are not useful in the clinical setting of probable RA but could be considered if a connective tissue disease such as lupus is in the differential diagnosis.
The diagnosis should also take into account disease activity (Table 18.20-1) and functional capacity (assessed, eg, with the Health Assessment Questionnaire score or EuroQol tools).
The American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) classification criteria are used: Table 18.20-2.
The most likely alternative diagnosis is crystalline arthritis with “pseudo-RA.” Other conditions that can mimic the joint pain of RA include spondyloarthritides encompassing psoriatic arthritis, and infective arthritis. Less commonly any of connective tissue diseases can present with arthritis; patients with connective tissue diseases develop arthralgia (pain but no inflammation in the joints) more frequently.
TreatmentTop
The treatment target is clinical remission according to one of several criteria. The ACR/EULAR criteria (Table 18.20-2) are frequently used. The target should be attained within 6 months; however, treatment should be modified if no improvement is observed after 3 months. The 2016 EULAR treatment algorithm: Figure 18.20-1.
1. DMARDs are the mainstay of therapy in RA, as they prevent or delay joint damage. They should be introduced promptly after the diagnosis of RA is established.Evidence 1Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of the intervention). Gaujoux-Viala C, Nam J, Ramiro S, et al. Efficacy of conventional synthetic disease-modifying antirheumatic drugs, glucocorticoids and tofacitinib: a systematic literature review informing the2013 update of the EULAR recommendations for management of rheumatoid arthritis. Ann Rheum Dis. 2014 Mar;73(3):510-5. doi: 10.1136/annrheumdis-2013-204588. Epub 2014 Jan 6. Review. PubMed PMID: 24395555; PubMed Central PMCID: PMC3932966. Nam JL, Ramiro S, Gaujoux-Viala C, et al. Efficacy of biological disease-modifying antirheumatic drugs: a systematic literature review informing the 2013 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis. 2014 Mar;73(3):516-28. doi: 10.1136/annrheumdis-2013-204577. Epub 2014 Jan 7. Review. Erratum in: Ann Rheum Dis. 2015 Jan;74(1):320. PubMed PMID: 24399231. Smolen JS, Breedveld FC, Burmester GR, et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis. 2016 Jan;75(1):3-15. doi: 10.1136/annrheumdis-2015-207524. Epub 2015 May 12. Review. PubMed PMID: 25969430; PubMed Central PMCID: PMC4717393. DMARDs are classified as follows:
1) Synthetic DMARDs:
a) Conventional DMARDs: MTX, leflunomide, sulfasalazine, gold salts, hydroxychloroquine.
b) Targeted DMARDs: Tofacitinib (Janus kinase [JAK] 3 and 1 inhibitor), baricitinib (JAK 1 and 2 inhibitor), upadacitinib (JAK 1 inhibitor).
2) Biologic agents (biologics; biologic DMARDs):
a) Innovator biologics, including anticytokine agents targeting tumor necrosis factor (TNF)-alpha (etanercept, adalimumab, infliximab, certolizumab, golimumab), interleukin 6 (IL-6) (tocilizumab), interleukin 1 (IL-1) (anakinra), and other biologic drugs targeting noncytokine pathways (abatacept, rituximab).
b) Biosimilar agents: For instance, biosimilar infliximab.
The choice of individual agents depends on disease activity and duration, prior treatment, prognostic factors (adverse prognostic factors include positive autoantibodies [RF and/or ACPAs, particularly in high titers], very high disease activity, early development of erosions), and comorbidities, as well as adverse effects and availability of the drugs (agents, dosage, contraindications, and treatment monitoring: Table 18.20-3).
In patients with active RA, start with MTX 15 mg once weekly, titrate up to the optimal dose (usually 20-25 mg/wk, with 25 mg/wk being the maximum; in the East Asian population, because of a lower body weight and possibly different pharmacogenetics, the maximum dose should be lower; Table 18.20-3) over 4 to 6 weeks (target dose might be a little lower in patients with adverse effects of MTX), and continue for ≥8 weeks in combination with folic acid 5 mg 24 hours after MTX. Folic acid counteracts some of the adverse effects (such as aphthous ulceration, nausea, hair loss) of the antimetabolite, MTX. In the case of contraindications to or intolerance of MTX, use oral leflunomide (10-20 mg daily) or oral sulfasalazine (up to 1.5 g bid); consider hydroxychloroquine in patients with low disease activity. In the initial treatment strategy, consider adding glucocorticoids for the shortest period possible (<3 months); the ACR suggests initiation of a conventional synthetic DMARD without short-term glucocorticoid use in DMARD-naive patients. Ensure prevention/treatment of osteoporosis (see Osteoporosis).
In the event that little improvement is observed within 3 months or the goals of treatment are not achieved within 6 months despite the use of optimal doses of a conventional synthetic DMARD or drug adverse effects develop (in the case of MTX intolerance start by changing the route of administration from oral to IM or subcutaneous), consider the following (see Figure 18.20-1):
1) In patients with poor prognostic factors (RF/ACPAs [especially in high titers], high disease activity, high swollen joint count, early joint damage, failure of ≥2 conventional synthetic DMARDs), add a biologic DMARD (TNF inhibitor [adalimumab, certolizumab, etanercept, golimumab, infliximab, or respective well-studied biosimilars], abatacept, IL-6 inhibitor [tocilizumab], or rituximab), or JAK inhibitor (tofacitinib, baricitinib, filgotinib, upadacitinib).Evidence 3Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of the intervention). Nam JL, Ramiro S, Gaujoux-Viala C, et al. Efficacy of biological disease-modifying antirheumatic drugs: a systematic literature review informing the 2013 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis. 2014 Mar;73(3):516-28. doi: 10.1136/annrheumdis-2013-204577. Epub 2014 Jan 7. Review. Erratum in: Ann Rheum Dis. 2015 Jan;74(1):320. PubMed PMID: 24399231.
2) In patients without poor prognostic factors change to or add a second conventional synthetic DMARD, optimally in combination with short-term glucocorticoids.Evidence 2Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of the intervention). Gaujoux-Viala C, Nam J, Ramiro S, et al. Efficacy of conventional synthetic disease-modifying antirheumatic drugs, glucocorticoids and tofacitinib: a systematic literature review informing the2013 update of the EULAR recommendations for management of rheumatoid arthritis. Ann Rheum Dis. 2014 Mar;73(3):510-5. doi: 10.1136/annrheumdis-2013-204588. Epub 2014 Jan 6. Review. PubMed PMID: 24395555; PubMed Central PMCID: PMC3932966.
Use all biologic DMARDs in combination with MTX (~10 mg/wk) or other conventional synthetic DMARDs. If a patient does not tolerate conventional synthetic DMARDs in combination therapy, IL-6 inhibitors or targeted synthetic DMARDs may be preferred. If the treatment target of low disease activity or remission has not been achieved within 3 to 6 months, consider switching to another biologic DMARD combined with a conventional synthetic DMARD. In rare cases (severe treatment-resistant RA or contraindications to conventional and/or biologic DMARDs) you may consider using azathioprine or cyclosporine (INN ciclosporin) (or, exceptionally, cyclophosphamide).
If a patient is in persistent (≥6 months) remission after having tapered glucocorticoids, you could discuss with the patient a careful reduction of the doses or increase of the use interval of a biologic DMARD, especially when a patient continues conventional DMARDs. Discontinuation of conventional DMARDs in patients in remission may also be considered but often results in a flare or exacerbation of RA and achieving a second remission is much more difficult.
2. Oral nonsteroidal anti-inflammatory drugs (NSAIDs) could be used for acute control of signs and symptoms of inflammation. In patients with contraindications to or intolerance of NSAIDs, use acetaminophen (INN paracetamol) and/or weak opioids (eg, tramadol).
3. Intra-articular glucocorticoids may be considered when the disease (or its exacerbation) involves only one or a few joints (injections to a single joint can be repeated not more often than every 3 months). Before injection exclude any other reasons for the exacerbation of joint manifestations, such as infection or crystal-associated arthropathy. Doses depend on the size of the joint: methylprednisolone, 40 to 80 mg; betamethasone, 0.8 to 4 mg; dexamethasone, 0.2 to 6 mg.
Rehabilitation should be part of treatment in every phase of the disease:
1) Physiotherapy increases muscle strength, improves physical functioning, prevents contractures and deformities, and prevents disability. There is no clear evidence for beneficial effects of other therapies, including electrotherapy, laser therapy, thermotherapy, cryotherapy, massage, and balneotherapy.
2) Psychological counseling.
Consider surgical treatment in the case of:
1) Severe pain and inflammation despite maximal intensity of medical treatment.
2) Joint destruction that limits the range of movement to an extent that causes significant disability.
Types of surgical interventions: synovectomy, reconstructive and corrective procedures, arthrodesis, and arthroplasty.
Synthetic DMARDs need not be stopped in the perioperative period; targeted DMARD tofacitinib should be stopped 7 days before surgeryEvidence 4Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due to indirectness and imprecision. Goodman SM, Springer B, Guyatt G, et al. 2017 American College of Rheumatology/American Association of Hip and Knee Surgeons Guideline for the Perioperative Management of Antirheumatic Medication in Patients With Rheumatic Diseases Undergoing Elective Total Hip or Total Knee Arthroplasty. Arthritis Rheumatol. 2017 Aug;69(8):1538-1551. doi: 10.1002/art.40149. Epub 2017 Jun 16. PubMed PMID: 28620948. It is recommended that biologic DMARDs are stopped within 2 half-lives prior to surgery and restarted after the surgical wound has healed with exact timing depending on the severity of underlying rheumatologic condition and probability of its deterioration.Evidence 5Low Quality of Evidence (low confidence that we know true effects of the intervention). Quality of Evidence lowered due to imprecision and indirectness. Goodman SM, Springer B, Guyatt G, et al. 2017 American College of Rheumatology/American Association of Hip and Knee Surgeons Guideline for the Perioperative Management of Antirheumatic Medication in Patients With Rheumatic Diseases Undergoing Elective Total Hip or Total Knee Arthroplasty. Arthritis Rheumatol. 2017 Aug;69(8):1538-1551. doi: 10.1002/art.40149. Epub 2017 Jun 16. PubMed PMID: 28620948.
Biologics should be stopped if the patient has an intercurrent infection.
Tables and FiguresTop
|
Disease activity measure |
Components |
Interpretation of results |
|
DASa |
In clinical practice DAS28 (including 28 joints: wrists, metacarpophalangeal joints, proximal interphalangeal joints, elbows, shoulders, knees) is used most commonly. Score is evaluated using a calculator that takes into account: 1) Number of swollen joints 2) Number of tender joints (including 28 joints: wrists, metacarpophalangeal joints, proximal interphalangeal joints, elbows, shoulders, knees) 3) ESR or CRP 4) Patient’s global assessment of disease activity using VAS (0-100) |
Score range: 0-9.4 Interpretation: <2.6: Remission ≤3.2: Low activity >3.2 and ≤5.1: Moderate activity >5.1: High activity Evaluation of treatment response: – Good: Change in activity ≥1.2 and low disease activity – Moderate: Change >0.6 and <1.2 and low or moderate disease activity, or change ≥1.2 and high or moderate disease activity – No response: Change <0.6 or <1.2 and high activity |
|
SDAI |
Takes into account same joints as DAS28 but does not require a calculator SDAI score = Number of tender joints + Number of swollen joints + Patient’s global assessment of disease activity using VAS (0-10 cm) + Physician’s global assessment of disease activity using VAS (0-10 cm) + Plasma CRP levels (0.1-10 mg/dL) |
Score range: 0.1-86 Interpretation: Score ≤3.3: Remission ≤11: Low activity >11 and ≤26: Moderate activity >26: High activity Evaluation of treatment response: – Significant improvement: Change >21 – Moderate improvement: Change 10-21 – No improvement: Change ≤9 |
|
CDAI |
Identical to SDAI but does not take CRP into account |
Score range: 0.1-76 Interpretation: Score ≤2.8: Remission ≤10: Low activity >10 and ≤22: Moderate activity >22: High activity |
|
ACR/EULAR remission criteria |
All must be fulfilled: – Tender joint count ≤1 – Swollen joint count ≤1 – Plasma CRP (mg/dL) ≤1 – Patient’s global assessment of disease activity using VAS (0-10) ≤1 or SDAI ≤3.3 |
Criteria recommended by EULAR for assessment of effectiveness of treatment in clinical practice |
|
In clinical practice, the CDAI is the most commonly performed measure of disease activity. Other measures are used mainly in research settings. | ||
|
ACR, American College of Rheumatology; CDAI, Clinical Disease Activity Index; CRP, C-reactive protein; DAS, Disease Activity Score; ESR, erythrocyte sedimentation rate; EULAR, European League Against Rheumatism; SDAI, Simplified Disease Activity Index; VAS, visual analog scale. | ||
|
Target population (who should be tested) Patients with: 1) ≥1 joint with definite clinical synovitis (swelling) 2) Synovitis not better explained by another diseasea The criteria are aimed at classification of newly presenting patients. In addition, patients with erosive disease typical of RAb or with long-standing disease, including those whose disease is inactive (with or without treatment), who based on retrospectively available data had previously fulfilled the 2010 criteria, should be classified as having RA. | |
|
Classification criteria for RA (score-based algorithm: add score of categories A-D; a score ≥6/10 is needed to classify a patient as having RA)c | |
|
A. Joint involvementd |
Score |
|
1 large jointe |
0 |
|
2-10 large joints |
1 |
|
1-3 small jointsf (with or without involvement of large joints) |
2 |
|
4-10 small joints (with or without involvement of large joints) |
3 |
|
>10 jointsg (including ≥1 small joint) |
5 |
|
B. Serology (≥1 test result is needed for classification)h |
Score |
|
Negative RF and negative ACPAs |
0 |
|
Low-positive RF or low-positive ACPAs |
2 |
|
High-positive RF or high-positive ACPAs |
3 |
|
C. Acute-phase reactants (≥1 test result is needed for classification) |
Score |
|
Normal CRP and normal ESR |
0 |
|
Abnormal CRP or abnormal ESR |
1 |
|
D. Duration of symptomsi |
Score |
|
<6 weeks |
0 |
|
≥6 weeks |
1 |
|
a Differential diagnosis may include conditions such as SLE, psoriatic arthritis, and gout. b Erosions (defined as disruptions of the bone cortex) revealed on radiographs of hands and feet in ≥3 individual joints among proximal interphalangeal joints, metacarpophalangeal joints, wrist joints (counted as 1 joint), and metatarsophalangeal joints. c Patients with a score <6/10 are not classifiable as having RA, however their status can be reassessed and the criteria may be fulfilled cumulatively over time. d Joint involvement refers to any swollen or tender joint on examination, which may be confirmed by imaging evidence of synovitis. Distal interphalangeal joints, first carpometacarpal joints, and first metatarsophalangeal joints are excluded from assessment (these are typically involved in osteoarthritis). e Large joints: Shoulders, elbows, hips, knees, and ankles. f Small joints: Metacarpophalangeal joints, proximal interphalangeal joints, second through fifth metatarsophalangeal joints, thumb interphalangeal joints, and wrists. g In this category ≥1 of the involved joints must be a small joint. Other joints can include any combination of large and additional small joints as well as other joints not specifically listed elsewhere (eg, temporomandibular, acromioclavicular, sternoclavicular). h Negative results refer to international unit values that are less than or equal to the ULN for the laboratory and assay. Low-positive results refer to international unit values that are higher than the ULN but 3 times the ULN for the laboratory and assay. High-positive results refer to international unit values that are 3 times the ULN for the laboratory and assay. i Duration of symptoms refers to the patient’s self-report of the duration of signs or symptoms of synovitis (eg, pain, swelling, tenderness) of joints that are clinically involved at the time of assessment, regardless of treatment status. | |
|
Source: Ann Rheum Dis. 2010;69(9):1580-8. | |
|
ACPA, anti-citrullinated peptide antibody; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; RA, rheumatoid arthritis; RF, rheumatoid factor; SLE, systemic lupus erythematosus; ULN, upper limit of normal. | |
|
Drug |
Dosage |
Contraindications |
Adverse reactions |
Monitoring |
|
Conventional (nonbiologic) DMARDs | ||||
|
Hydroxychloroquine |
200 mg PO once daily or bid |
Retinal diseases; visual impairment; renal failure; porphyria; psoriasis; G6PD deficiency; untreated chronic hepatitis B or C with Child-Pugh class C |
Retinal (macular) damage reversible after drug discontinuation; rash; abdominal pain, diarrhea, appetite loss, nausea; other (very rare): myopathy, blurred vision, visual impairment, abnormal cutaneous/mucosal pigmentation, peripheral neuropathy |
Ophthalmic exam (fundoscopy and visual fields): before starting treatment, then after 5 years (or 1 year if there are risk factors for retinopathy: macular disease, low eGFR, tamoxifen use, and hydroxychloroquine dose >5 mg/kg/d or chloroquine dose >2.3 mg/kg/d), with further follow-up every 12 months. There is a maximum lifetime dose of 1000 g, which is reached after 7 years on 400 mg/d |
|
Leflunomide |
10-20 mg PO once daily |
Infectiona; leukopenia <3×109/L; thrombocytopenia <50×109/L; myelodysplasia; lymphoproliferative disorder treated in last ≤5 years; liver diseaseb,c,d; pregnancy and breastfeeding; severe or moderate renal impairment |
Diarrhea, abdominal pain, nausea; rash; alopecia; liver damage; kidney damage; hypertension; teratogenicity (effective contraception necessary). In case of complications, drug has to be discontinued; elimination may be accelerated using cholestyramine (8 g tid for 11 days) or activated charcoal (50 g qid for 11 days). In women planning pregnancy and men planning to become fathers, levels of metabolite of leflunomide should be measured several times following accelerated elimination |
CBC: before starting treatment, then every 2-4 weeks in initial 3 months of treatment, then every 2-3 months; after 6 months of treatment repeat every 3 months or less frequently. Serum creatinine: every 2 weeks until target drug dose is established, then every month. Serum ALT and AST: as above; in case of sustained increase in AST/ALT >3 × ULN, drug should be discontinued and liver biopsy considered to assess liver damage |
|
Methotrexate |
15-25 mg PO, IM, or SC once weekly; titrate dose to a max 25 mg; folic acid (5 mg/wk) or leucovorin (INN folinic acid) to be used concomitantly to prevent adverse effects (cytopenia, mouth ulceration, nausea) |
As above + interstitial pneumonitis/pulmonary fibrosis; CrCl <30 mL/min |
Elevated serum liver enzyme levels, liver fibrosis and cirrhosis (very rare); risk factors: alcohol consumption, obesity, diabetes, hepatitis B and C; pancytopenia due to bone marrow suppression and complications (dose-dependent); oral ulcers (in 30% of patients); nausea within 24-48 h of drug administration; interstitial lung disease (2%-6% regardless of dose and treatment duration); teratogenicity (effective contraception necessary); methotrexate should be discontinued (in both women and men) 3 months before planned conception; milder adverse effects (due to folic acid deficiency): mucositis, alopecia, GI disturbances |
As above + chest x-ray before starting treatment (results of studies performed in the previous year are acceptable) and in course of treatment if cough or dyspnea develop |
|
Sulfasalazine |
1.5 g PO bid (dose should be titrated up) |
Hypersensitivity to sulfonamides and salicylate; after ileostomy; liver diseaseb,c,e; renal failure; porphyria; G6PD deficiency; breastfeeding; considered safe in pregnancy |
Majority of adverse effects occur within first few months of treatment and can be avoided by starting with low dose and titrating up gradually: loss of appetite, dyspepsia, nausea, vomiting, abdominal pain (30%); headache, vertigo/dizziness; fever; allergic skin (urticaria, photosensitivity) and joint reactions; hemolytic anemia (in patients with G6PD deficiency), very rarely aplastic anemia; granulocytopenia (1%-3%) may occur at any time of treatment (most commonly in first 3 months); elevated serum ALT/AST; interstitial lung disease (rare) |
CBC, serum ALT, AST, and creatinine: as above |
|
Biologic DMARDs: TNF inhibitors | ||||
|
Adalimumab |
20-40 mg SC every 1-2 weeks |
Infectiona; viral hepatitisc,e; pregnancy, breastfeeding; heart failure (NYHA class III/IV and EF ≤50%); multiple sclerosis or other demyelinating disease; lymphoproliferative disease treated in last ≤5 yearsf |
Severe infections (including opportunistic infections); positive autoantibodies including ANAs, anti-dsDNA, anticardiolipin, and antichimeric; rarely drug-induced lupus (in which case treatment should be discontinued); cytopenias (mainly leukopenia); demyelination syndromes, optic neuritis (very rare) with symptoms resolving after drug discontinuation; reactivation of HBV infection; elevated serum ALT/AST |
Before starting: chest x-ray and tuberculin skin test/IGRA test, CBC, serum ALT/AST and creatinine, tests for viral hepatitis; pneumococcal (periodic), influenza (annual), and hepatitis B (in patients at risk of infection) vaccinations recommended; live vaccines contraindicated but could be administered prior to starting DMARD or biologic treatment; in course of treatment patients should be monitored for symptoms of infection; in women mammography is recommended before starting treatment |
|
Etanercept |
25 mg SC twice a week or 50 mg/wk |
As above |
As above | |
|
Infliximab |
3-10 mg/kg IV, repeated after 2 and 6 weeks, then every 8 weeks, or 3-5 mg/kg every 4 weeks |
As above |
As above | |
|
Certolizumab |
200 mg SC bid, repeated after 2 and 4 weeks, followed by maintenance dose 200 mg every 2 weeks |
As above |
As above |
As above |
|
Golimumab |
50 mg SC once a month |
As above |
As above |
As above |
|
Other biologic DMARDs | ||||
|
Abatacept |
IV infusion over 30 min; body weight <60 kg: 500 mg, 60-100 kg: 750 mg, >100 kg: 1 g; subsequent doses to be administered 2 and 4 weeks after first infusion, then every 4 weeks |
Infectiona; viral hepatitisc,e; pregnancy and breastfeeding |
Severe infections; probably causes progressive multifocal leukoencephalopathy (very rare) |
Before starting: chest x-ray and tuberculin skin test/IGRA test, CBC, serum ALT/AST and creatinine, tests for viral hepatitis; pneumococcal (periodic), influenza (annual), and hepatitis B (in patients at risk of infection) vaccinations recommended; live vaccines contraindicated; in course of treatment patients should be monitored for symptoms of infection; in women mammography is recommended before starting treatment |
|
Rituximab |
1g IV, 2 doses at 14-day interval; can be repeated every 6 months |
Infectiona; viral hepatitisc,e; pregnancy and breastfeeding |
Allergic reactions, infections, probably causes progressive multifocal leukoencephalopathy (very rare), reactivation of HBV infection |
As above + plasma immunoglobulin levels |
|
Tocilizumab |
8 mg/kg IV every 4 weeks or 162 mg SC weekly |
Infectiona; ALT/AST >5 × ULN; viral hepatitisc,g; neutropenia |
Infections, neutropenia, and thrombocytopenia, elevated ALT/AST (in particular during concomitant use of potentially hepatotoxic drugs, eg, DMARDs), dyslipidemia, intestinal perforation in patients with diverticulitis (infrequent) |
As above (drug may suppress acute-phase reaction so patients should be very carefully monitored for features of infection) + ALT/AST every 4-8 weeks for first 6 months of treatment, then every 3 months; CBC after 4-8 weeks of treatment, then repeat when necessary |
|
Targeted DMARDs | ||||
|
Tofacitinib |
5 mg PO bid |
Malignancy, current infection, pregnancy and breastfeeding |
Headache, diarrhea, bacterial infections, herpes zoster infection |
TB skin prick test and chest x-ray; CBC, ALT, AST, creatinine, and lipids; monitor for infection and malignancy |
|
Baricitinib |
2 mg PO daily |
As tofacitinib |
As tofacitinib |
As tofacitinib |
|
a Active bacterial infection, TB (active or latent in patients not receiving anti-TB prophylaxis), active infection with varicella zoster or herpes simplex virus, active severe fungal infection (in the case of biologic agents probably also febrile viral upper respiratory tract infection and poorly healing infected skin ulcers). b ALT and/or AST levels >2 × ULN. c Acute hepatitis B or C. d Chronic hepatitis B or C (regardless of treatment status and severity of liver disease). e Chronic hepatitis B (unless treated and with Child-Pugh class A; sulfasalazine can be used in both class A and B) or chronic hepatitis C associated with Child-Pugh class B or C (etanercept is recommended as potentially safe in patients with chronic hepatitis C). f Rituximab is recommended in patients with RA qualifying for a biologic agent and a history of treated lymphoproliferative malignancy or skin melanoma (anytime), as well as treated within the last 5 years nonmelanoma skin cancers or solid malignancies. g Evidence is scarce regarding safety of tocilizumab in chronic viral hepatitis. h Loading dose may be used to speed up the onset of effect; however, it is common practice to avoid it because of the risk of diarrhea and adherence issues. It is not recommended when leflunomide is used in combination therapy. | ||||
|
ANA, antinuclear antibody; ALT, alanine aminotransferase; AST, aspartate aminotransferase; bid, 2 times a day; CBC, complete blood count; CrCl, creatinine clearance; DMARD, disease-modifying antirheumatic drug; EF, ejection fraction; eGFR, estimated glomerular filtration rate; G6PD, glucose-6-phosphate dehydrogenase; GI, gastrointestinal; IM, intramuscular; IV, intravenous; HBV, hepatitis B virus; IGRA, interferon gamma release assay; INN, international nonproprietary name; NYHA, New York Heart Association; PO, oral; qid, 4 times a day; RA, rheumatoid arthritis; SC, subcutaneous; TB, tuberculosis; tid, 3 times a day; TNF, tumor necrosis factor; ULN, upper limit of normal. | ||||
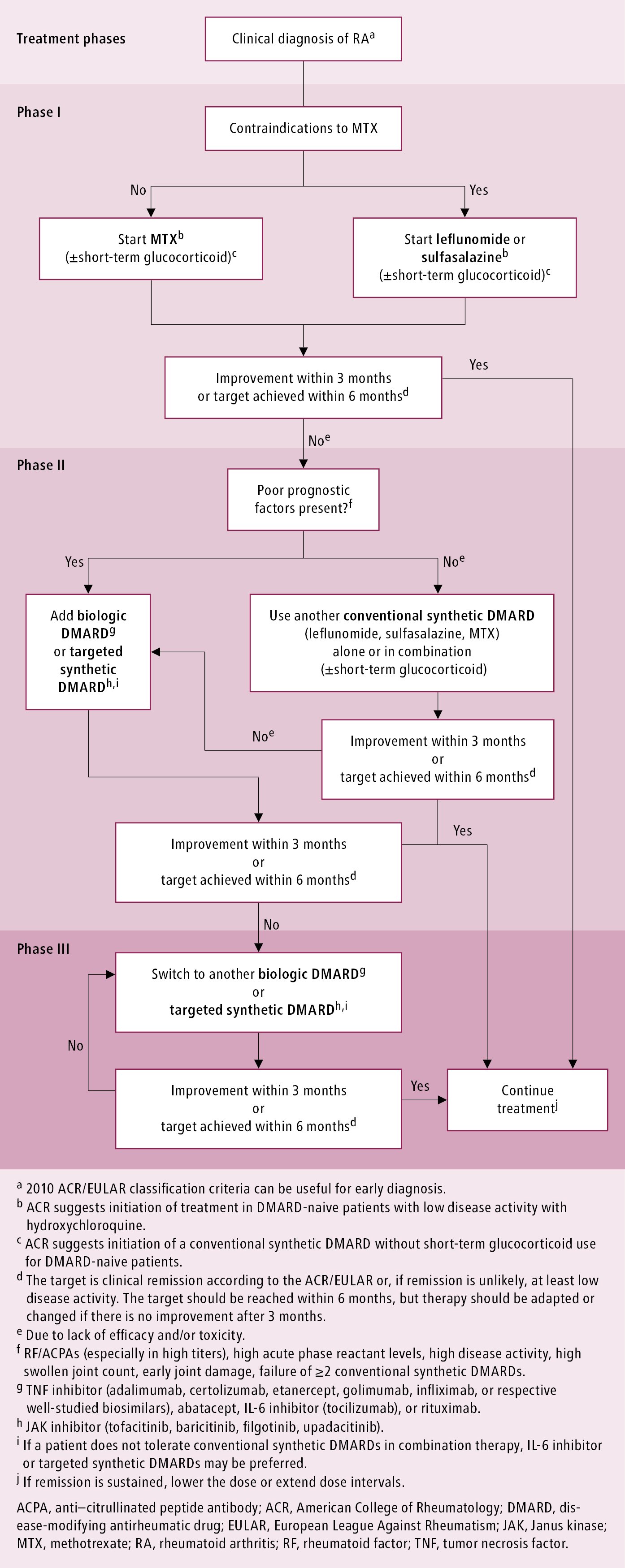
Figure 18.20-1. Management algorithm for rheumatoid arthritis. Based on Ann Rheum Dis. 2020;79:685-699.