Definition, Etiology, PathogenesisTop
1. Etiologic agent: Protozoans of the genus Plasmodium: P falciparum, P vivax, P ovale, P malariae, and P knowlesi. Additionally, cases of human P cynomolgi infection have been reported in Malaysia and Cambodia, and symptomatic infections with P brasilianum, in Venezuela (P cynomolgi, P brasilianum, and P knowlesi are simian parasites).
2. Reservoir and transmission: The reservoirs for plasmodia are humans or monkey macaques (for P knowlesi). Malaria is transmitted by mosquitoes (Anopheles spp), which contract the infection when biting an infected human or macaque. Ten to 100 sporozoites (invading human form of plasmodium) are sufficient to cause infection. They are carried by blood to the liver and mature in hepatocytes into hepatic schizonts. After 1 to 2 weeks (up to 4 weeks for P malariae), large numbers of another form of parasite, merozoites (~10,000), are released from infected hepatic cells containing schizonts and invade red blood cells (RBCs). The next forms—the trophozoites—that develop from merozoites, are initially ring shaped and then mature into erythrocytic schizonts, which cause RBC rupture and release of further merozoites. The cycle is repeated every ~48 hours (P falciparum, P ovale, and P vivax) to ~72 hours (P malariae), or every ~24 hours for P knowlesi. Some merozoites develop into sexual gametocytes, which infect mosquitoes during the blood meal, and the sexual stage of the parasite’s life cycle begins leading to development of sporozoites.
3. Pathogenesis: The release of merozoites from ruptured RBCs triggers episodes of fever and other clinical manifestations of the disease. Severity depends on the extent of invasion of RBCs; for P falciparum infection the percentage of infected RBCs may exceed 10%. Massive parasitemia in P falciparum infection is a serious threat: The parasites release substances that cause pronounced systemic inflammatory response due to overproduction of proinflammatory cytokines by the host’s immune cells (cytokine storm) and lead to RBC adhesion to the vascular endothelium, which results in organ ischemia and hypoxia due to impaired blood flow, and to coagulopathy symptoms. This may result in severe, rapidly progressing central nervous system (CNS) damage, multiorgan failure, or even death. A growing number of severe cases of malaria caused by P vivax have been reported over the recent years. For P ovale and P vivax it may take several months to years until the onset of symptoms or relapse from hypnozoites in the liver.
4. Risk factors for severe infection: Staying in areas endemic for Plasmodium spp, activities favoring exposure to vector bites. The risk of severe malaria is the highest in infants, young children, and pregnant women living in areas endemic for P falciparum and in nonimmune individuals living in temperate climates who travel to endemic regions.
5. Incubation and contagious period: Usually 12 to 35 days; or 12 to 14 days for P falciparum. In very rare cases the onset of symptoms may occur as soon as 7 days following the mosquito bite or after several months (particularly in P vivax and P malariae infections).
Of note, some types of parasite (P ovale and P vivax) may form hypnozoites (from sporozoites invading liver cells), which are dormant forms of the parasite in the liver, and may cause relapse months or years later.
EpidemiologyTop
Malaria is widespread in the tropics (Figure 10.9-3). More than 90% of cases occur in sub-Saharan Africa. There were 229 million of malaria cases reported in 87 endemic countries in 2019, and 8638 imported cases reported in the European Union/European Economic Community over the same period.
Clinical Features And Natural HistoryTop
Fever is the most typical symptom of malaria. The temperature increases rapidly, remains elevated (even >40 degrees Celsius), and is accompanied by severe chills. The patient is anxious or agitated. After several hours the fever decreases or resolves, excessive sweating occurs, and the patient falls asleep. Febrile episodes recur every ~48 hours (P ovale and P vivax) to ~72 hours (P malariae) or every ~24 hours (P knowlesi). Fever associated with P falciparum infection is usually continuous or irregular.
Fever may be accompanied by weakness, diarrhea, headache, myalgia, cough, jaundice, nausea, and vomiting. Symptoms and diagnostic criteria of severe malaria (usually due to P falciparum infection): Table 10.9-1. In P falciparum malaria progression to severe disease with multiorgan dysfunction and pronounced systemic inflammatory response (cytokine storm) may be rapid and unpredictable.
Adults staying in endemic areas with intense Plasmodium spp transmission for years often have mild symptoms or asymptomatic disease.
DiagnosisTop
Travelers with fever who return from regions endemic for malaria should always undergo diagnostic testing for malaria.
1. Identification of the etiologic agent:
1) Microscopic examination of Giemsa-stained capillary blood smear (both thin and thick smears) allows for identification of the parasite species on the basis of its morphologic features and for assessment of the extent of parasitemia.
2) Rapid immunochromatographic tests detecting malarial antigens allow for distinguishing P falciparum from other Plasmodium species.
3) High-sensitivity molecular tests (polymerase chain reaction [PCR]) are performed exclusively in reference laboratories, but they do not assess the extent of parasitemia.
4) Loop-mediated isothermal amplification (LAMP) tests.
5) Serologic studies allowing for detection of specific antibodies are not used in the diagnostic workup of patients with suspected malaria, but they are usually performed to confirm past infection.
2. Other common findings include anemia, thrombocytopenia, elevated alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels, hyperbilirubinemia. Laboratory indicators of severe P falciparum infection: Table 10.9-1.
Dengue, other viral hemorrhagic fevers, chikungunya, influenza, meningitis, pneumonia, infectious endocarditis, sepsis, typhoid fever and paratyphoid fevers, leptospirosis, rickettsial infections, African trypanosomiasis, babesiosis.
TreatmentTop
Treatment of the Underlying Condition
1. Uncomplicated P falciparum infection in endemic regions and in adult travelers: The World Health Organization (WHO) recommends artemisinin-based combination therapy (ACT) for 3 days. Oral ACT is not routinely available in nonendemic countries; in such a situation, in adults, it is recommended to use 4 tablets of atovaquone/proguanil daily for 3 days or a combination of oral doxycycline plus oral quinine for 7 days, or a combination of oral clindamycin plus oral quinine for 7 days.
2. Uncomplicated non–P falciparum malaria: Use chloroquine or ACT if the patient is resistant to chloroquine. In P vivax and P ovale infections, primaquine and tafenoquine are also used (available in the United States, not recommended by the WHO), which are active against hypnozoites.
3. Severe malaria, regardless of the Plasmodium species (most commonly P falciparum): The first-line treatment is artesunate IV or IM. If artesunate is not available, use artemether or quinine. In patients treated with parenteral antimalarial agents for ≥24 hours, switch to oral therapy (ACT) if possible. Oral ACT may not be available in nonendemic countries; after 24 hours options are either to switch to 4 tablets of atovaquone/proguanil daily for 3 days or to use a combination of oral doxycycline/clindamycin plus quinine for 7 days.
4. Malaria imported to a malaria-free country: Treatment with locally available antimalarial drugs should be started as soon as possible. In adult travelers with uncomplicated malaria (P falciparum), the WHO recommends ACT. Atovaquone/proguanil may also be used.
5. Fever in travelers to endemic regions: Consider standby emergency treatment (SBET) if:
1) There is no possibility to obtain medical care within 24 hours.
2) The patient is planning frequent, short travels to malaria-risk areas (eg, job-related travels of airline personnel).
3) Travels to regions with very low risk of multidrug-resistant P falciparum malaria (eg, some regions of Southeast Asia and Amazonia), where the risk of adverse effects of chemoprophylaxis exceeds the risk of infection.
For SBET recommend ACT according to the local recommendations for mild malaria. Provide the traveler who plans to follow this management with detailed advice on the clinical manifestations, course of malaria, and treatment principles, and recommend urgent medical consultation following SBET.
6. Dosage of antimalarial agents: Table 10.9-2. Of note, ACT and parenteral therapies are not easily available is some countries, especially with low prevalence of disease where treatment is usually conducted in specialized tropical medicine or infectious diseases centers.
Severe malaria usually requires comprehensive supportive treatment, such as blood product transfusion, hemodialysis for acute kidney injury, respiratory support, correction of electrolyte disturbances and hypoglycemia, anticonvulsant and antipyretic treatment, and antibiotics in the case of concomitant bacterial infection.
Special ConsiderationsTop
P falciparum infection in pregnancy is associated with increased risk of severe disease and death in the pregnant woman, miscarriage or premature delivery, intrauterine fetal death, low birth weight, and perinatal death of the infant. The treatment of choice in severe malaria in pregnant patients is artesunate IV or IM. The WHO recommends oral quinine and clindamycin (dosage: see Table 10.9-2) to treat uncomplicated malaria in the first trimester of pregnancy. Oral artesunate with clindamycin or ACT can be used as an alternative if first-line agents are not available. ACT is recommended in the second and third trimesters.
In regions with high rates of transmission of P falciparum, the WHO recommends intermittent preventive treatment in pregnancy (IPTp) with sulfadoxine/pyrimethamine from the second trimester.
Because of the risk associated with malaria during pregnancy, advise nonimmune pregnant patients residing in malaria-free areas to avoid travels to regions endemic for P falciparum.
PrognosisTop
The mortality rate in patients with imported malaria is about 0.5% to 3%. In adequately treated patients with severe infection, the mortality rate may be as high as 20%. Permanent sequelae of malaria, most frequently neuropsychiatric (eg, memory impairment, mood disturbances, psychosis), develop in <5% of adults infected with P falciparum. Very rarely invasions of other species lead to life-threatening complications, such as splenic rupture in P vivax infection.
PreventionTop
1. Vaccination: Starting from October 6, 2021, the WHO recommends the RTS,S/AS01 vaccine to prevent malaria caused by P falciparum in children aged ≥5 months who live in sub-Saharan Africa and other regions with moderate or high transmission of the parasite.
2. Chemoprophylaxis in persons traveling to tropics.
General principles of malaria chemoprophylaxis in travelers: The principal aim is to reduce the risk of malaria caused by P falciparum and minimize the risk of severe disease and death. The effectiveness of appropriate regular chemoprophylaxis in preventing symptomatic infection is >95% for P falciparum and ~80% for P vivax infections. The majority of antimalarial drugs do not protect against late relapses caused by P vivax or P ovale hypnozoites. The only drugs with activity against hypnozoites are primaquine and tafenoquine. Tafenoquine is approved for malaria prophylaxis in the United States, but it is not recommended by the WHO.
When selecting malaria chemoprophylaxis consider the drug resistance of plasmodia in the region of destination, duration of stay, and the patient’s medical history and tolerance of previously used antimalarial agents (Table 10.9-3). Chemoprophylaxis should be used according to the regimen of the specific drug and started 1 to 7 days before arrival in the region at risk for malaria transmission, and continued throughout the stay and for 1 to 4 weeks after leaving the endemic region (Table 10.9-4).
Chemoprophylaxis during long-term travel: The risk for malaria increases with exposure to mosquito bites and duration of stay in an endemic area. The majority of adverse effects of drugs used as chemoprophylaxis manifest within the initial weeks of therapy. Long-term chemoprophylaxis in areas with high rates of P falciparum infection depends on the individual assessment of the benefits and risks of this approach and should be recommended at least at the beginning of the patient’s stay in the tropics, when they gain experience in the use of nonspecific prophylaxis and orient themselves in access to local medical care. The maximum acceptable duration of treatment with individual agents used as malaria prophylaxis has not been specified. The WHO allows the use of atovaquone with proguanil, doxycycline, and mefloquine for several months if well tolerated by the patient. After 5 years of chloroquine treatment at a preventive dose, the risk of retinopathy increases (regular ophthalmologic monitoring is necessary). The widespread drug resistance restricts the use of chloroquine also in this group of travelers.
In selected situations consider periodic chemoprophylaxis, which is to be used in specific seasons of the year or in selected regions on the travel route. This approach should be preceded by a detailed analysis of the seasonal variability and intensity of local malaria transmission in the countries of destination. However, in many of the regions in the tropics, this assessment is impossible due to limited availability of epidemiologic data.
Malaria prophylaxis in pregnant travelers: Travels to malaria endemic areas should be definitely avoided in pregnancy due to the risks associated with P falciparum infection in pregnant women and the fetus. If the travel is necessary, recommend strict observance of malaria prophylaxis principles by the patient. The chemoprophylaxis options are limited in this group. Doxycycline is contraindicated in pregnancy (category D); atovaquone with proguanil is not recommended due to lack of data concerning safety for the fetus; chloroquine can be used in pregnancy, but its usefulness is limited by drug resistance of P falciparum in the tropics.
1. In endemic regions: Eradication of mosquito breeding grounds and ensuring mass mosquito bite protection (eg, indoor residual spraying).
2. In individuals traveling to the tropics: Advise the patient prior to travel on the risk of malaria in the region of destination, signs, symptoms, need for urgent diagnostic workup in the case of fever when staying in the region with active malaria transmission or upon return, and mosquito bite protection measures. Avoiding contact with the vector is the key prevention method during long travels to endemic areas.
Tables and FiguresTop
|
Symptoms or syndromes |
Definition |
|
Impaired consciousness |
In adults: GCS score <11 |
|
Prostration |
Generalized weakness: inability to sit, stand, or walk without assistance
|
|
Multiple convulsions |
>2 episodes within 24 h
|
|
Severe anemia |
Hematocrit <20% or blood hemoglobin <7 g/dL with concurrent parasitemia >10,000/microL |
|
Jaundice |
Serum bilirubin >3.0 mg/dL (> 50 micromol/L) with concurrent parasitemia >100,000/microL |
|
Acute kidney injury |
Serum creatinine >3.0 mg/dL (265 micromol/L) or BUN >20 mmol/L |
|
Pulmonary edema or acute respiratory failure |
Pulmonary edema confirmed by radiography or arterial oxygen saturation <92% on room air, with respiratory rate >30/min, often paradoxical movements of the chest and crepitus on auscultation |
|
Hypoglycemia |
Serum glucose <40 mg/dL (2.2 mmol/L) |
|
Shock |
– Compensated: capillary refill time >3 s or cold distal parts of lower limbs – Decompensated: systolic arterial pressure <80 mm Hg with evidence of impaired perfusion |
|
Bleeding |
Gum bleeding, epistaxis, GI bleeding |
|
Hyperparasitemia |
Parasitemia >10% in endemic countries; in nonendemic countries, >5% is considered as severe malaria |
|
Acidosis |
Base deficit >8 mEq/L or serum HCO3– <15 mmol/L or lactic acid >5 mmol/L |
|
a Severe P vivax malaria is defined as P falciparum malaria but with no parasite density thresholds. Severe P knowlesi malaria is defined as P falciparum malaria, with 2 differences: – P knowlesi hyperparasitemia: Parasite density >100,000/microL. – Jaundice and parasite density: >20,000/microL. | |
|
Adapted from 2021 World Health Organization guidelines. | |
|
BUN, blood urea nitrogen; GCS, Glasgow Coma Scale; GI, gastrointestinal; WHO, World Health Organization. | |
|
Indication |
Agent |
Dosage |
|
Severe malaria (most frequently caused by P falciparum); use parenteral treatment for ≥24 h, until oral administration is possible, then start ACT |
Artesunate (first-line agent) |
2.4 mg/kg IV or IM in 3 doses over 24 h: at 0, 12, and 24 h; then switch to once daily |
|
Artemether |
IM, starting dose of 3.2 mg/kg; followed by 1.6 mg/kg once daily | |
|
Quinine (salt) |
20 mg/kg in IV infusion followed by 10 mg/kg every 8 h | |
|
Uncomplicated malaria caused by P falciparum (ACTs) |
Artemether + lumefantrine |
Body weight ≥35 kg: 80 mg + 480 mg PO bid for 3 days; the first 2 doses should be administered at an 8-h interval |
|
Artesunate + amodiaquine |
Body weight ≥36 kg: 200 mg + 540 mg PO once daily for 3 days | |
|
Artesunate + mefloquine |
Body weight ≥30 kg: 200 mg + 440 mg PO once daily for 3 days | |
|
Dihydroartemisinin + piperaquine |
Body weight ≥36 kg and <60 kg: 120 mg + 960 mg PO once daily for 3 days; body weight ≥60 kg and <80 kg: 160 mg + 1280 mg PO once daily for 3 days; in persons with body weight ≥80 kg: 200 mg + 1600 mg PO once daily for 3 days | |
|
Artesunate + SP |
Body weight ≥50 kg: 200 mg of artesunate PO for 3 days + 1500 mg/75 mg SP PO in a single dose on day 1 | |
|
Artesunate + pyronaridine |
Body weight ≥45 kg and <65 kg: 180 mg + 540 mg PO once daily for 3 days; body weight ≥65 kg: 240 mg + 720 mg PO once daily for 3 days | |
|
Reduction of P falciparum malaria transmission following treatment in endemic regions with low transmission rates |
Primaquine |
0.25 mg/kg PO, in a single dose |
|
Uncomplicated malaria in travelers returning from the tropics |
ACT |
As above |
|
Atovaquone + proguanil |
1000 mg + 400 mg PO once daily for 3 days | |
|
Uncomplicated malaria caused by other Plasmodium species |
ACT |
As above |
|
Chloroquine |
10 mg/kg PO once daily for 2 days, 5 mg/kg on day 3 | |
|
Uncomplicated malaria in the first trimester of pregnancy |
Quinine (salt) + clindamycin |
650 mg PO tid + 10 mg/kg PO bid for 7 days |
|
P vivax and P ovale relapse prevention after acute infection treatment |
Primaquine |
0.25-0.5 mg/kg PO once daily for 14 days |
|
Adapted from: World Health Organization 2021 guidelines. | ||
|
ACT, artemisinin-based combination therapy; bid, two times a day; PO, orally; SP, sulfadoxine/pyrimethamine; tid, three times a day; WHO, World Health Organization. | ||
|
Risk of malaria in the region of destination |
Recommended prophylaxis |
Remarks |
|
Very low risk of Plasmodium spp infection |
Mosquito bite precautions only |
Very few regions in Central and East Asia and in North Africa |
|
Risk of P vivax malaria only |
Mosquito bite precautions + chemoprophylaxis (chloroquine or atovaquone/proguanil, doxycycline, or mefloquine)a |
Some Caribbean islands, some regions in Central and South America and Central Asia |
|
Risk of P falciparum malaria, plasmodium resistance to chloroquine |
Mosquito bite precautions + chemoprophylaxis (atovaquone/proguanil, doxycycline, or mefloquine)a |
The majority of malaria-endemic regions worldwide |
|
Risk of P falciparum malaria + documented mefloquine resistance |
Mosquito bite precautions + chemoprophylaxis (atovaquone/proguanil or doxycycline)a |
Southeast Asia: some regions of Myanmar, Thailand, and Cambodia |
|
a In regions with very low risk of P falciparum infection and documented multidrug resistance, the use of mosquito bite precautions only and SBET is permitted. | ||
|
Adapted from WHO International Travel and Health 2012 (updated 2017). | ||
|
SBET, standby emergency treatment; WHO, World Health Organization. | ||
|
Agent |
Dosage and prophylactic regimen |
Remarks |
|
Atovaquone/proguanil |
Atovaquone 250 mg/proguanil hydrochloride 100 mg PO every day; initiate prophylaxis 1-2 days before arrival to an endemic area; continue throughout stay and 7 days thereafter |
Effectively prevents malaria in the majority of endemic regions and areas in Southeast Asia with multidrug resistance; its mechanism of action allows for early discontinuation of chemoprophylaxis after travel |
|
Chloroquine |
A single dose of chloroquine diphosphate 500 mg PO every week; initiate prophylaxis ≥1 week before arrival to an endemic area; continue throughout stay and 4 weeks thereafter |
Due to common drug resistance of P falciparum, chloroquine is currently considered ineffective prophylaxis in the majority of endemic regions, including Africa; use of chloroquine is limited to certain countries in Latin America, Middle East, and China; long-term use may cause damage to the retina |
|
Doxycycline |
100 mg PO every day; initiate prophylaxis 1-2 days before arrival to an endemic area; continue throughout stay and 4 weeks thereafter |
Effectively prevents malaria in the majority of endemic regions and areas in Southeast Asia with multidrug resistance; due to risk of phototoxicity, avoid excessive sunlight exposure during agent intake and apply UV protection products |
|
Mefloquine |
A single dose of 250 mg PO every week; initiate prophylaxis ≥1 week before arrival to an endemic area; continue throughout stay and 4 weeks thereafter |
Limited use due to drug resistance of P falciparum in Southeast Asia and risk of neuropsychiatric adverse effects; can be used by pregnant women |
|
PO, oral; WHO, World Health Organization. |
||
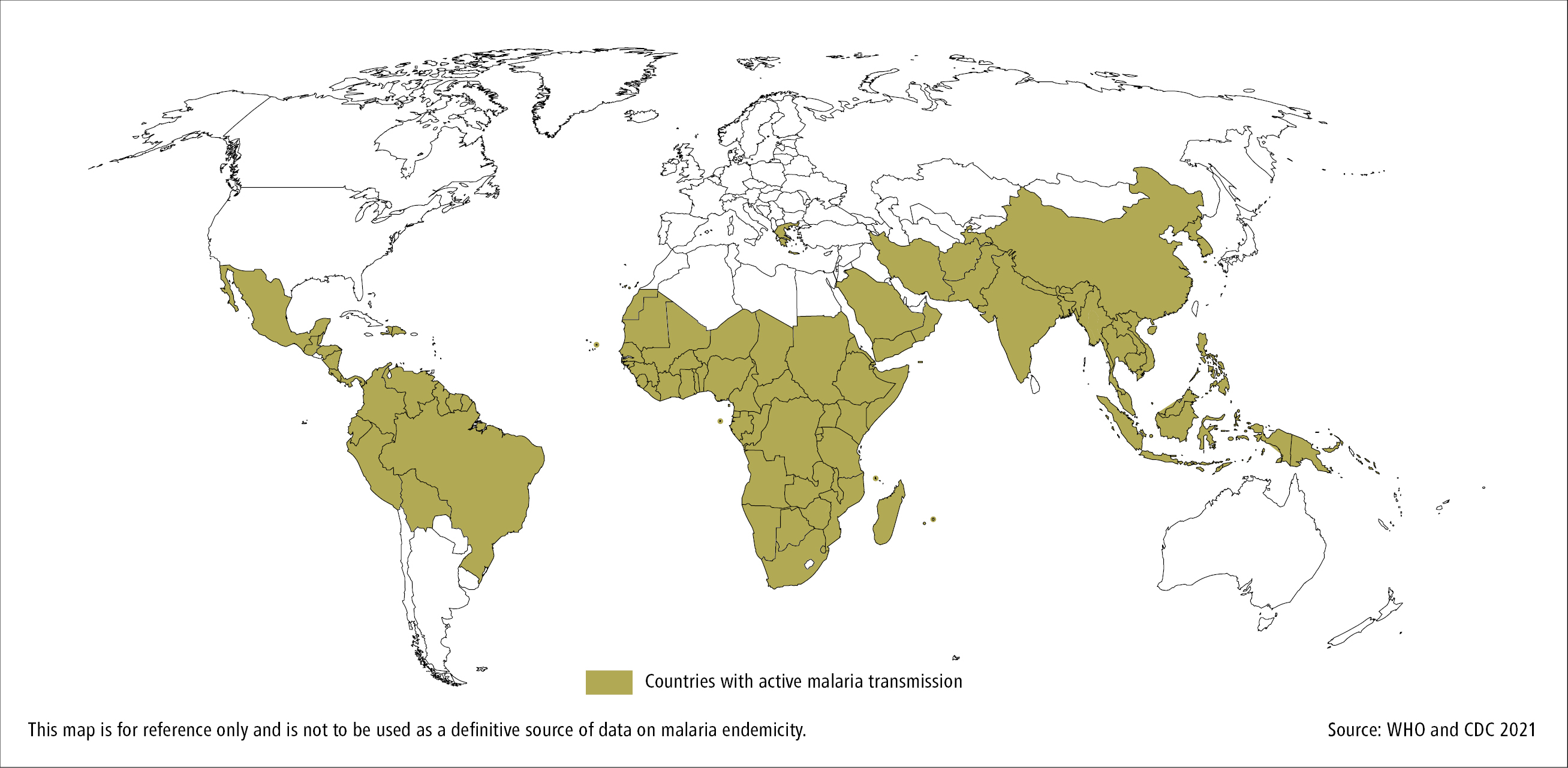
Figure 10.9-3. Geographic coverage of plasmodia in the Western and Eastern hemispheres.