Etiology and PathogenesisTop
1. Etiologic agent: Taenia solium cestode. After ingestion of infective eggs by the host, larvae (oncospheres) escape the eggs, enter the bloodstream, and localize in organs, particularly in the brain, rarely in the eyeball, muscles, or subcutaneous tissue, where they form cysticerci (1-2 cm in diameter). Viable cysticerci can survive in tissues for many years, slowly undergoing calcification and, finally, dying.
2. Reservoir and transmission: Humans are definitive hosts. Cysticercosis develops after ingestion of food contaminated with eggs from T solium proglottids excreted with feces by the same host (autoinfection—a life-threating consequence of taeniasis) or by a different host.
3. Risk factors: Invasion of an adult cestode (risk of autoinfection), work in a laboratory (T solium proglottids are highly contagious biologic material), travel to endemic countries (eg, Mexico, Ecuador, Peru, Bolivia, India, Bali).
4. Incubation and contagious period: From several months to >10 years. Individuals with cysticercosis are not contagious to contacts, unless they are hosts for an adult cestode.
EpidemiologyTop
Cysticercosis is endemic in many Latin American, Asian, and African countries.
Clinical Features and Natural HistoryTop
Clinical presentation depends on the location and number of cysticerci (most often in the brain and eye).
1. Neurocysticercosis (most common): Cysticerci localize mainly on the surface of the cerebral cortex or within the base of the brain, ventricles, or white matter and cause local inflammatory lesions with accompanying edema. The disease is asymptomatic in ~50% of cases. In the remaining cases it is manifested by signs and symptoms typical for brain tumor (depending on lesion location): Epileptic seizures (eg, convulsions), altered mental status, manifestations of increased intracranial pressure, hydrocephalus, altered behavior (personality disorders, unstable emotions), mental disorders (cognitive dysfunction, delusional syndromes, memory loss, dementia), and other manifestations of focal central nervous system (CNS) damage (ataxia, dysarthria). In rare cases of spinal cord involvement, various movement (paralysis, paresis) and sensation disorders may occur, including transverse myelitis. Prognosis is poor (high risk of persistent, long-term neurologic complications, depending on lesion location).
2. Ocular cysticercosis: Cysticerci localize primarily in the subretinal space, vitreous body, or anterior eyeball. The key manifestations of the disease include various vision disorders due to extensive inflammatory and compression lesions.
3. Intramuscular cysticercosis: Multiple calcifications (sometimes palpable) or skeletal muscle pseudohypertrophy. The disease is typically asymptomatic.
4. Subcutaneous cysticercosis: Numerous palpable subcutaneous nodules.
5. Rare locations of cysticerci: Myocardium, thyroid, lungs, peritoneal cavity. Cysticerci cause local inflammation, which is usually asymptomatic.
DiagnosisTop
Information about the patient’s stay in the endemic area aids in diagnosis; lack of information about a history of past taeniasis or habit of ingesting raw or undercooked pork has no diagnostic significance.
1. Identification of the etiologic agent:
1) Serologic studies (enzyme-linked immunosorbent assay [ELISA]; results should be confirmed by Western blot assay) are used to confirm the diagnosis by detecting specific IgG antibodies in serum (or cerebrospinal fluid [CSF] in neurocysticercosis). Studies may yield negative results in the case of single or calcified (nonviable) cysticerci.
2) Histologic examination of excised tissue: Identification of a scolex, hooklets, and fragments of the vesicular wall of a cysticercus in tissue.
3) Identification of a coexisting adult form of T solium in the small intestine (microscopic examination of proglottids).
2. Other tests:
1) Complete blood count (CBC): Eosinophilia (in reaction to antigens released by the parasite; absent in some cases).
2) Imaging (computed tomography [CT], magnetic resonance imaging [MRI], or ultrasonography): Characteristic findings (Figure 10.9-1) are suggestive of cysticercosis; numerous focal lesions of various size and density or echogenicity (ranging from hypodense or echogenic to completely calcified lesions), which vary depending on the development stage and viability of cysticerci. Patients in nonendemic countries may present with single focal lesions. Calcified T solium cysticerci in the brain or muscles can be visualized on radiography.
3) CSF examination: Pleocytosis with lymphocytic (in some cases eosinophilic) predominance, high protein and immunoglobulin concentrations, decreased glucose levels.
4) Ophthalmic examination is necessary in all patients to exclude ocular involvement.
Differential diagnosis depends on the location of cysticerci and includes primary and metastatic cancers (including lymphoma), vascular malformations, tuberculosis, systemic mycoses, cystic or alveolar echinococcosis, toxoplasmosis, and brain abscess.
TreatmentTop
Treatment of the Underlying Condition
Conservative (antiparasitic agents) or surgical treatment should be used depending on the number and location of cysticerci.
1. Antiparasitic agents are indicated in active, symptomatic cysticercosis and contraindicated if cysticerci are localized in the eye or ventricles of the brain:
1) Oral albendazole 7.5 mg/kg every 12 hours for 10 to ≥14 days.
2) Alternatively oral praziquantel 40 mg/kg/d in 3 divided doses, every 8 hours for 10 to 14 days.
According to the Infectious Diseases Society of America (IDSA) guidelines, combination therapy with albendazole and praziquantel is preferred in patients with neurocysticercosis if the number of cysticerci is >2.
In conditions where prolonged therapy and monitoring are not feasible or the patient may be lost to follow-up, consider 1-day therapy with oral praziquantel at a dose of 75 mg/kg/d (3 doses of 25 mg/kg every 2 h; both regimens probably have similar efficacy).
Depending on the severity of invasion and location of cestode larvae, treatment for up to 30 days or repeated antiparasitic therapy may be indicated after several months. Effective treatment of T solium infection (see Tapeworm Infections) prevents recurrence of cysticercosis caused by autoinfection.
Antiparasitic agents may exacerbate clinical manifestations; thus, in neurocysticercosis, initiate hospital treatment and use glucocorticoids (see Symptomatic Treatment); delay treatment in the case of hydrocephalus and cerebral edema. Due to the risk of cerebral edema, do not use antiparasitic agents if cysticerci are present in the eyeball or in the vicinity of the ventricles of the brain or a large number of cysticerci is observed in the CNS (multiple focal lesions). As per the IDSA guidelines, the treatment approach in patients with spinal cord cysticercosis should be selected on a case-by-case basis, considering, among others, clinical manifestations, lesion location, and the level of experience in the surgical treatment of cysticercosis.
For single, degenerating (characteristic hyperdense lesions on CT or MRI), or nonviable (calcified) cysticerci, consider withholding antiparasitic treatment (due to high risk of inefficacy and exacerbation of symptoms—inflammation and neurologic manifestations related to degeneration of parasites). Patients should be treated in specialized centers. Individuals treated with albendazole for >14 days should be monitored for hepatotoxicity and leukopenia.
2. Surgical treatment is indicated for single cysticerci localized in the ventricles or at the base of the brain (those lesions do not respond to antiparasitic agents), spinal cord cysticercosis, and ocular cysticercosis (antiparasitic treatment is contraindicated due to risk of complications resulting from degeneration of parasites). Lesions are excised or enucleation of the eye is performed. In the case of internal hydrocephalus, ventriculoperitoneal shunting is necessary.
1. Glucocorticoids should be used in patients with major edema or cell infiltrates to reduce inflammatory response to parasites degenerating as a result of antiparasitic treatment. No optimal regimens have been established. Some experts propose IM dexamethasone 10 to 20 mg/d in 2 to 4 divided doses for the first 4 days of antiparasitic treatment or oral prednisone 50 mg tid/wk during long-term treatment.
2. Antiepileptic drugs and agents reducing intracranial pressure (see Stroke) should be used in patients with complications of neurocysticercosis (seizures and increased intracranial pressure).
Follow-UpTop
It is recommended to regularly assess serum concentration of specific antibodies to cysticerci (every 6 months), absolute and relative eosinophilia during antiparasitic treatment, and head MRI scans in order to monitor treatment efficacy (frequency of examinations depends on the number and location of cysticerci as well as risk of edema and increase in intracranial pressure).
ComplicationsTop
Complications depend on the location of cysticerci:
1) Neurologic: Epilepsy, hydrocephalus, increased intracranial pressure.
2) Ocular: Retinal detachment.
PreventionTop
None.
1. Maintaining basic hand hygiene.
2. Early diagnosis and treatment of human taeniasis (see Tapeworm Infections).
3. Veterinary inspection and adequate thermal processing of pork.
FiguresTop
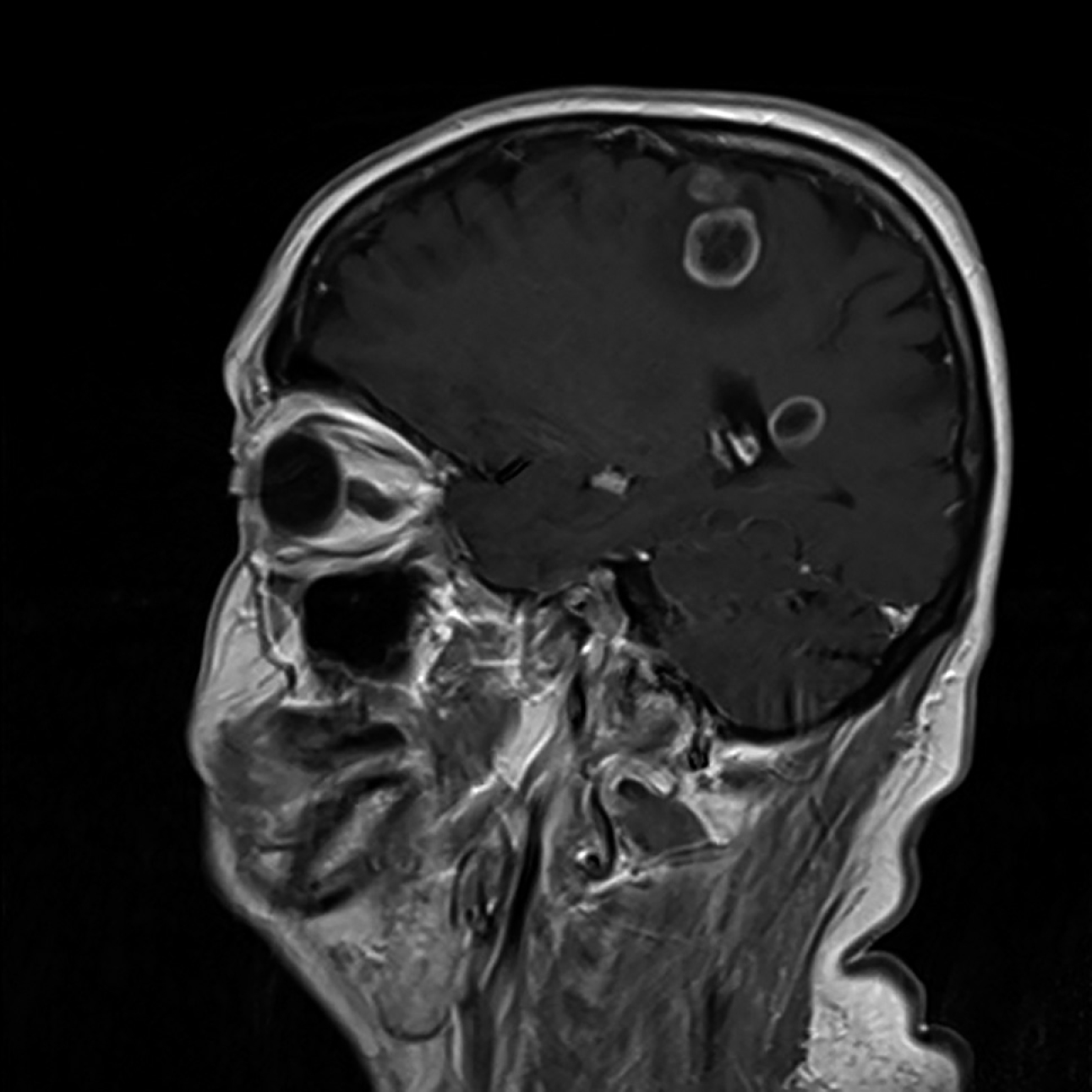
Figure 10.9-1.
MRI of the head: Solitary larvae (cysticerci) of Taenia solium in brain tissue.