Baumgartner H, De Backer J, Babu-Narayan SV, et al; ESC Scientific Document Group. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur Heart J. 2021 Feb 11;42(6):563-645. doi: 10.1093/eurheartj/ehaa554. PMID: 32860028.
Stout KK, Daniels CJ, Aboulhosn JA, et al. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019 Apr 2;139(14):e637-e697. doi:10.1161/CIR.0000000000000602. PMID: 30586768.
Ventricular septal defects (VSDs) are the most common congenital heart disease seen at birth (excluding bicuspid aortic valves), accounting for 30% to 40% of all defects.
Classification of VSDs based on their location:
1) Perimembranous VSD (most common, accounting for ~80% of all cases): Located in the membranous septum.
2) Muscular/trabecular VSD (up to 15%-20% of cases): Completely surrounded by muscle. These could be multiple (“Swiss cheese” defects).
3) Outlet VSD (~5% of cases): Located underneath the semilunar valves.
4) Inlet/atrioventricular canal: Directly inferior to the atrioventricular valves. A component of the atrioventricular septal defect.
Clinical Features and Natural HistoryTop
1. Symptoms: Small VSDs are asymptomatic. Symptoms of a larger VSD include exertional dyspnea, reduced exercise tolerance, and palpitations. The development of pulmonary vascular disease (which eventually leads to shunt reversal and Eisenmenger syndrome) causes a significant limitation of the patient’s activity.
2. Signs: In patients with small VSDs the apical impulse is normal in location and size. In those with large VSDs the apical impulse is located inferiorly and laterally, secondary to left ventricle (LV) dilatation. This is also the case in patients with hemodynamically important aortic regurgitation (associated with aortic cusp prolapse) or significant mitral regurgitation (associated with inlet-type VSDs). A loud holosystolic murmur in the fourth left intercostal space with a systolic thrill is the predominant sound heard during auscultation (the smaller the defect, the louder the murmur and the more pronounced the presence of the associated thrill). Muscular VSDs often have murmurs that are not holosystolic due to the systolic compression of the defect. In patients with large-volume shunts there may be a diastolic apical rumble. With the development of Eisenmenger syndrome the systolic murmur disappears, the second heart sound becomes distinctly accentuated, and a diastolic murmur of pulmonary regurgitation (Graham Steell murmur) may appear. A low-frequency diastolic decrescendo murmur along the sternal border should make one suspect the presence of aortic regurgitation, which can be related to aortic cusp prolapse.
3. Natural history: Moderate left-to-right shunts lead in adulthood to LV volume overload, congestive heart failure, and subsequently to the development of Eisenmenger syndrome and shunt reversal. The defect may close spontaneously at any age, although this rarely occurs in adults (<10%) and applies to perimembranous and muscular defects only.
DiagnosisTop
Diagnosis is largely made on the basis of echocardiography. However, muscular VSDs located at the apex, even when multiple, may be missed on routine echocardiographic evaluation. Differential diagnosis includes other lesions that lead to holosystolic murmurs, such as mitral regurgitation or tricuspid regurgitation.
1. Electrocardiography (ECG): In a large VSD the features of LV and left atrial hypertrophy are observed. When severe pulmonary vascular disease develops (see Eisenmenger syndrome), there is evidence of right ventricular hypertrophy and right atrial enlargement.
2. Chest radiography (Figure 3.7-1): In patients with a significant VSD there are features of increased pulmonary perfusion (shunt vascularity), and there could also be evidence of LV and left atrial enlargement. With the development of pulmonary hypertension, varying degrees of pulmonary artery enlargement are seen.
3. Echocardiography (Figure 3.7-2): Echocardiography can identify the location and size of VSD(s) as well as assess cardiac remodeling caused by the shunt. Calculation of the pulmonary (Qp) to systemic (Qs) flow ratio can be performed in individuals with good imaging windows. An important component of an echocardiographic evaluation is the assessment of pulmonary artery systolic pressures as well as evaluation for possible coexisting defects.
4. Cardiac catheterization: Although not routinely performed, in some patients it may be necessary to assess the hemodynamic significance of the VSD (shunt ratio calculation), main pulmonary artery pressures, as well as pulmonary vascular resistance. In individuals with significantly elevated pulmonary artery pressures on noninvasive imaging, preoperative cardiac catheterization is necessary to evaluate pulmonary vascular reactivity. In patients being forwarded for surgical closure of the defect who are at risk of coronary artery disease, assessment of coronary arteries is performed via cardiac catheterization.
TreatmentTop
1. Patients with normal pulmonary artery pressures and a small shunt (with less than moderate aortic regurgitation): No treatment is necessary. Current guidelines do not recommend routine endocarditis prophylaxis in these patients.
2. Surgical treatment is recommended in patients who are:
1) Symptomatic but do not have Eisenmenger syndrome.
2) Asymptomatic but have LV volume overload (more than mild LV dilation) that is not related to other etiologies.
3. Surgical treatment may also be considered in patients with:
1) A history of infective endocarditis.
2) Progressive aortic regurgitation due to aortic cusp prolapse.
3) Pulmonary hypertension, if the Qp:Qs ratio is >1.5 and pulmonary artery pressures or pulmonary vascular resistance are <2/3 of the respective systemic values (in baseline conditions, after the administration of a pulmonary artery vasodilator, or following targeted treatment for pulmonary hypertension).
Follow-UpTop
Patients after surgery with no residual hemodynamic disturbances and with cardiovascular symptoms only require a periodic specialist follow-up (a 5-year interval is adequate). However, patients with LV dysfunction, pulmonary hypertension, residual shunt, aortic regurgitation, right ventricular outflow obstruction, or LV outflow obstruction require a more regular (annual) evaluation (both clinical and imaging) in specialized clinics.
ComplicationsTop
Infective endocarditis (reported up to 2 in 1000 patient-years), aortic regurgitation requiring surgery, paradoxical embolism (during intraventricular pacing), and complete heart block (rare).
FiguresTop
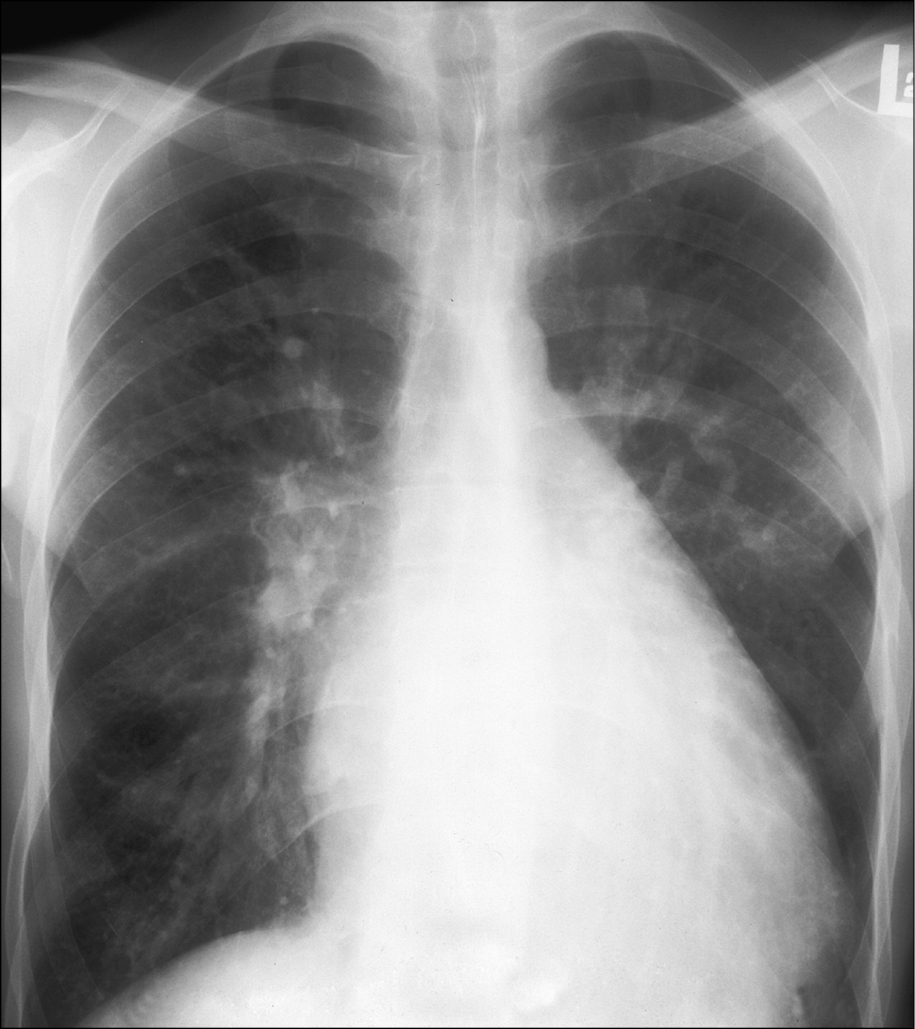
Figure 3.7-1. Chest radiography of a patient with ventricular septal defect (VSD): dilatation of the left ventricle, main pulmonary artery, and pulmonary arteries; features of increased pulmonary blood flow; narrow ascending aorta. Figure courtesy of Dr Piotr Hoffman.
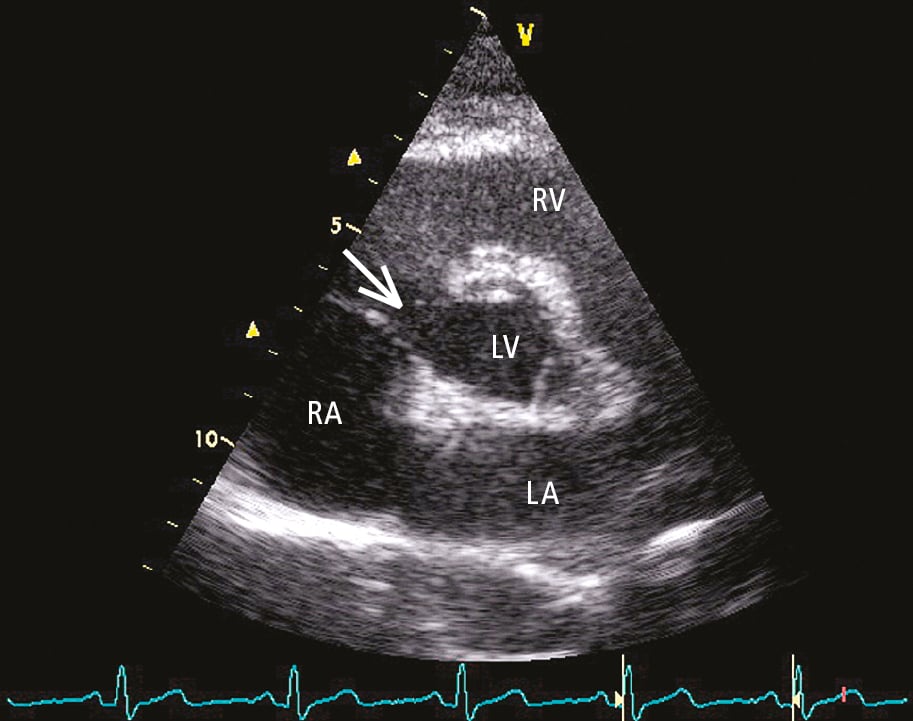
Figure 3.7-2. Transthoracic echocardiography (TTE), parasternal short-axis view: perimembranous ventricular septal defect (VSD) (arrow). LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle. Figure courtesy of Dr Piotr Hoffman.