Delgado V, Ajmone Marsan N, de Waha S, et al. 2023 ESC Guidelines for the management of endocarditis. Eur Heart J. 2023;44(39):3948-4042. doi:10.1093/eurheartj/ehad193
Fowler VG, Durack DT, Selton-Suty C, et al. The 2023 Duke-International Society for Cardiovascular Infectious Diseases Criteria for Infective Endocarditis: Updating the Modified Duke Criteria. Clin Infect Dis. 2023;77(4):518-526. doi:10.1093/cid/ciad271
Hubers SA, DeSimone DC, Gersh BJ, Anavekar NS. Infective Endocarditis: A Contemporary Review. Mayo Clin Proc. 2020;95(5):982-997. doi:10.1016/j.mayocp.2019.12.008
Baddour LM, Wilson WR, Bayer AS, et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation. 2015;132(15):1435-1486. doi:10.1161/CIR.0000000000000296
Definition, Etiology, PathogenesisTop
Infective endocarditis (IE) is an infection of the endocardium most frequently involving the heart valves, although the disease can occur in other areas covered with endocardium such as ventricles, atria, or endothelium of vessels (eg, in patients with coarctation of the aorta). IE can manifest in patients with cardiovascular implantable electronic devices (CIEDs) through affecting the electrode leads, valves, or endocardial surface. Most frequently IE affects the mitral and aortic valves. Less frequently the tricuspid valve may be involved. IE can involve >1 valve, and the proportion of these patients varies in reported literature. IE is preceded by bacteremia, which can last from <2 weeks (80% of patients) to several months (in particular in patients with IE involving a prosthetic valve).
Etiologic agents:
1) Bacteria (>90% of cases). Most frequent pathogens:
a) Staphylococci (Staphylococcus aureus, the most common cause of IE in high-income countries; Staphylococcus epidermidis; coagulase-negative staphylococci).
b) Streptococci (viridans group streptococci; until recently the most frequent cause of native valve infections).
c) Enterococci (most commonly Enterococcus faecalis).
d) The HACEK group of fastidious gram-negative organisms (Haemophilus spp [including H parainfluenzae, the most likely cause among HACEK organisms], Aggregatibacter [previously known as Actinobacillus] spp, Cardiobacterium hominis, Eikenella corrodens, and Kingella spp).
e) Non-HACEK gram-negative bacteria.
f) Mixed bacterial etiology is frequently found among IV drug users.
2) Fungi (<1%).
3) Very rare: Chlamydia, rickettsia, or mycoplasma.
Depending on criteria definitions for IE, etiology cannot be established in 5% to 20% of patients.
Blood culture–negative infective endocarditis (BCNIE) refers to a form of IE where the usual blood culture methods fail to identify any causative microorganism. This condition presents significant challenges in both diagnosis and treatment. Several factors influence the likelihood of BCNIE, such as antibiotic administration prior to diagnostic evaluation, availability of diagnostic tests, and local epidemiology. Based on this, BCNIE can be divided into several categories. Most often BCNIE occurs due to previous antimicrobial use in cases of IE with typical pathogens (eg, staphylococci, streptococci, and enterococci), highlighting the crucial need to collect blood cultures before starting antimicrobial treatment. However, in the literature and practice BCNIE more commonly refers to cases of IE caused by fastidious organisms and intracellular organisms. Etiologic agents in patients with BCNIE can be identified through serology, molecular methods (polymerase chain reaction [PCR], sequencing), or tissue biopsy, depending on the type of suspected organism. Microorganisms that cause BCNIE include:
1) Coxiella burnetii.
2) Bartonella spp.
3) Mycoplasma pneumoniae.
4) Brucella spp.
5) Legionella spp.
6) Fungi (eg, Aspergillus spp).
7) Tropheryma whipplei.
Diseases and conditions predisposing to native valve endocarditis (NVE): Risk factors for IE include poor dentition or oral infection, history of rheumatic disease, mitral valve prolapse with regurgitation, hypertrophic cardiomyopathy, valvular or congenital heart disease (particularly affecting the aortic valve, eg, bicuspid aortic valve; coarctation of the aorta), degenerative cardiac lesions, and chronic hemodialysis. Intravenous drug use (IVDU) as well as presence of intravascular devices (eg, indwelling central venous catheters) and CIEDs are significant risk factors additionally associated with the involvement of right-sided heart valves (~10% of all IE cases).
Prosthetic valve endocarditis (PVE) accounts for 10% to 30% of all cases of IE. It most frequently develops within 5 to 6 weeks of surgery. IE occurring within 12 months of surgery is considered a postoperative complication. In the first 2 months after surgery PVE is most frequently caused by S aureus followed by coagulase-negative staphylococci (mainly methicillin-resistant strains) and Candida spp. PVE occurring 2 to 12 months after surgery is caused by a blend of nosocomial and community-acquired infections. In PVE developing >1 year after surgery etiologic agents are similar to those seen in NVE.
Cardiac device–related infective endocarditis (CDRIE) is most frequently caused by coagulase-negative staphylococci and S aureus, the most important risk factor being recent manipulation of the device.
Clinical FeaturesTop
IE presentation is highly variable and differential diagnosis is often broad, sometimes leading to delay in establishing diagnosis. Symptom onset may be rapid (acute) or occur over weeks to months (subacute). IE is manifested mainly by nonspecific symptoms, including high-grade fever with chills or prolonged low-grade fever (the most frequent feature), malaise, weakness, arthralgia, myalgia, loss of appetite, weight loss, headache, and nausea.
1. Left-sided endocarditis may also cause symptoms associated with:
1) A new or changing regurgitation murmur over the affected valve.
2) Features of heart failure, including pulmonary edema in patients with no prior history of valvular disease.
3) Conduction abnormalities (first- or second-degree atrioventricular block, bundle branch block, or complete heart block).
4) Rarely large vegetations leading to functional mitral stenosis.
5) Embolic phenomena (most frequently associated with S aureus), including:
a) Central nervous system (CNS) symptoms (30%-40% of patients; hemiparesis, aphasia; behavioral changes in those with microembolism).
b) Rarely intracranial hemorrhage due to ruptured mycotic aneurysm.
c) Renal, splenic, or mesenteric embolism, which may lead to adynamic ileus resulting in abdominal pain or back pain.
d) Coronary artery embolism (rare) manifesting as chest pain.
e) Ocular disturbances associated with retinal artery embolism.
f) Peripheral vascular and inflammation symptoms (petechiae on skin and under nail plates [Figure 3.10-1], Osler nodes [painful red nodules located mainly on fingers and toes due to deposition of immune complexes], Janeway lesions [painless hemorrhagic lesions on palms and soles], Roth spots [retinal hemorrhages with pale centers]).
g) Splenomegaly and hepatomegaly (more frequent in patients with long-standing IE).
2. Right-sided endocarditis may also cause symptoms associated with:
1) Pulmonary embolism, with associated cough and pleuritic chest pain (caused by septic pulmonary emboli). This is the most common manifestation. It may be associated with pulmonary infarcts, pleural effusion, and empyema.
2) Murmur caused by tricuspid or pulmonary regurgitation: Often absent or seen in advanced disease.
3) Features of right ventricular failure in patients with long-standing IE.
4) Often recurrent right-sided IE in the case of IVDU, frequently in the absence of other predisposing heart conditions.
IE must always be excluded in patients with embolism and fever.
DiagnosisTop
The following studies should be performed in every patient with suspected IE.
1. Blood cultures are critical for the diagnosis of IE and treatment planning. Obtain ≥3 blood culture sets from separate venipuncture sites before starting antimicrobial treatment, with the first and last samples being drawn ≥1 hour apart, regardless of body temperature. Each sample should contain 10 mL of blood collected to an aerobic tube and another 10 mL collected to an anaerobic tube. Mark the order form as suspected IE. Blood culture fails to identify the causative pathogen in up to 20% of patients. In all patients undergoing cardiac surgery, and particularly those with negative results of previous cultures, perform specimen cultures and pathologic examination. If needed, use molecular identification methods, to identify the etiologic agent.
2. Serologic studies: Perform serologies and PCR in the case of suspected IE with negative blood cultures (see Definition, Etiology, Pathogenesis, above).
3. Echocardiography: In suspected endocarditis it is important to evaluate for vegetations (mobile echogenic structures attached to the endocardium or intracardiac prosthetic material; Figure 3.10-2), valvular damage (regurgitation of the infected valve due to vegetations, leaflet perforation [Figure 3.10-3], or rupture of chordae tendineae), and perivalvular complications (abscess [Figure 3.10-4], pseudoaneurysm, intracardiac fistula).
In all patients without prosthetic valves with IE suspected on the basis of clinical criteria, transthoracic echocardiography (TTE) should be performed.Evidence 1 Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of the intervention). Mügge A, Daniel WG, Frank G, Lichtlen PR. Echocardiography in infective endocarditis: reassessment of prognostic implications of vegetation size determined by the transthoracic and the transesophageal approach. J Am Coll Cardiol. 1989 Sep;14(3):631-8. PubMed PMID: 2768712. Lindner JR, Case RA, Dent JM, Abbott RD, Scheld WM, Kaul S. Diagnostic value of echocardiography in suspected endocarditis. An evaluation based on the pretest probability of disease. Circulation. 1996 Feb 15;93(4):730-6. PubMed PMID: 8641002. Habib G, Badano L, Tribouilloy C, et al; European Association of Echocardiography. Recommendations for the practice of echocardiography in infective endocarditis. Eur J Echocardiogr. 2010 Mar;11(2):202-19. doi: 10.1093/ejechocard/jeq004. PubMed PMID: 20223755. Bonzi M, Cernuschi G, Solbiati M, Giusti G, Montano N, Ceriani E. Diagnostic accuracy of transthoracic echocardiography to identify native valve infective endocarditis: a systematic review and meta-analysis. Intern Emerg Med. 2018 Sep;13(6):937-946. doi: 10.1007/s11739-018-1831-0. Epub 2018 Mar 15. PubMed PMID: 29546685. In those with a low clinical probability of IE and a negative TTE result (provided that good-quality images are obtained), IE is unlikely and another diagnosis should be considered. If good-quality TTE images cannot be obtained, transesophageal echocardiography (TEE) should be performed. TEE should be also performed in:
1) Patients with a high clinical probability of IE and a negative TTE result.
2) Patients with suspected IE and a prosthetic valve or intracardiac device.
3) Patients with suspected IE affecting the aortic valve.
4) Patients with IE and significant valvular regurgitation.
5) Patients with a TTE result suggestive of IE (except for those with IE affecting right-sided native heart valves and unequivocal TTE findings). In the case of a negative TEE result and reasonable suspicion of IE, repeat TEE after 5 to 7 days.
Although it is the mainstay for diagnosis of IE, echocardiography has its limitations (it cannot reliably differentiate between active and healed IE, and TTE and TEE sensitivity and specificity are not 100%). As such, results must be interpreted in the context of clinical presentation.
4. Laboratory tests: There are no biochemical parameters that have been proven to be sensitive or specific for the diagnosis of IE. However, laboratory evaluation is part of standard workup of IE. IE has been associated with elevated erythrocyte sedimentation rate (ESR), increased levels of C-reactive protein (CRP) and fibrinogen, leukocytosis with neutrophilia (most frequent in acute IE), anemia (usually normocytic and normochromic), hematuria, and minor proteinuria.
5. Electrocardiography: Nonspecific findings and conduction abnormalities. The presence of heart block or conduction delay, which may manifest initially as a prolonged PR interval, can provide an important clue to paravalvular extension of infection.
6. Chest radiography: Nonspecific findings, which may include congestive heart failure findings and signs of pulmonary complications. It may help exclude alternative causes of fever and systemic symptoms.
7. Multislice computed tomography (CT) and magnetic resonance imaging (MRI): CT provides a valuable addition to echocardiography in the diagnosis of perivalvular lesions: abscesses, pseudoaneurysms, and fistulas. It may be also used in patients with prosthetic valves. Multislice CT is useful in the anatomical assessment of the aortic valve (eg, leaflet perforation) and aorta and in the diagnosis of pulmonary embolism in patients with right-side IE, metastatic abscesses (eg, in the spleen), and CNS-related emboli. CT has lower sensitivity than MRI but is more available.
8. Nuclear imaging: Nuclear imaging techniques, such as 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) have demonstrated potential as a supplementary method for selected patients in the case of difficulties in the diagnosis and management of IE, especially in those with prosthetic valves.Evidence 2Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). Low Quality of Evidence (low confidence that we know true effects of the intervention). Quality of Evidence lowered due to indirectness and imprecision. Rouzet F, Chequer R, Benali K, et al. Respective performance of 18F-FDG PET and radiolabeled leukocyte scintigraphy for the diagnosis of prosthetic valve endocarditis. J Nucl Med. 2014 Dec;55(12):1980-5. doi: 10.2967/jnumed.114.141895. Epub 2014 Nov 13. PMID: 25453046. Duval X, Le Moing V, Tubiana S, et al.; AEPEI-TEPvENDO study group. Impact of Systematic Whole-body 18F-Fluorodeoxyglucose PET/CT on the Management of Patients Suspected of Infective Endocarditis: The Prospective Multicenter TEPvENDO Study. Clin Infect Dis. 2021 Aug 2;73(3):393-403. doi: 10.1093/cid/ciaa666. PMID: 32488236. Mahmood M, Kendi AT, Ajmal S, et al. Meta-analysis of 18F-FDG PET/CT in the diagnosis of infective endocarditis. J Nucl Cardiol. 2019 Jun;26(3):922-935. doi: 10.1007/s12350-017-1092-8. Epub 2017 Oct 30. PMID: 29086386.
The diagnosis of IE can be made in a patient with sepsis or generalized infection and objective features of endocardial involvement. Relevant diagnostic terminology includes:
1) Definite IE: Table 3.10-1.
2) Possible IE: Table 3.10-1.
3) Active IE:
a) Positive blood cultures or positive intraoperative specimens.
b) Intraoperative confirmation of features of endocarditis.
c) Unfinished course of antimicrobial treatment for IE.
4) Relapse: IE caused by the same microorganism within <6 months of a confirmed episode of IE.
5) Reinfection: IE caused by the same microorganism after >6 months of a previous episode of IE or caused by a different microorganism.
6) CDRIE: CDRIE is difficult to differentiate from local infection of the device. It should be suspected in the case of fever of unknown origin in a patient with a CIED. The key diagnostic procedures are echocardiography (TEE has superior sensitivity and specificity but TTE should be performed first) and blood cultures.
Other causes of fever (see Fever of Unknown Origin), systemic connective tissue disease, malignancy, rheumatic fever.
Causes of false-positive echocardiography results: Sterile intracardiac thrombus or tumor resembling vegetations, sterile valvular vegetations (eg, in Libman-Sacks endocarditis in the course of systemic lupus erythematosus, or less frequently in Behçet syndrome, carcinoid, or acute rheumatic fever).
TreatmentTop
The determination of empiric and even targeted management, including considerations of surgery, should take into account numerous factors related to a specific patient and a particular epidemiological milieu. Previous history of endocarditis, previous antibiotic treatments, presence of intravascular devices, severity of disease and occurrence of complications, presence of antimicrobial allergies, comorbidities (especially renal), location and structure of vegetations, and local epidemiology/resistance patterns are all crucial. The complexity of choices for both empiric and targeted therapy is illustrated by the fact that 2023 European Society of Cardiology (ESC) guidelines for IE management have 95 pages, the majority of which are devoted to management choices (doi:10.1093/eurheartj/ehad193; the last parallel clinical practice guideline from the American Heart Association [AHA] was published in 2015). Our suggestions below, based mostly on ESC guidelines, may be oversimplified in some cases but nevertheless present reasonable starting alternatives. In general, infectious disease specialist input, if only feasible, should be obtained. Surgical consultation may be required.
Overall, treatment of IE requires eradication of infection and prolonged parenteral and bactericidal therapy. Initial therapy and stabilization of the patient in the hospital is recommended, with involves stabilization using rapidly bactericidal IV antibiotics and cardiac interventions if indicated. This first phase of treatment can last up to 2 weeks. Outpatient therapy for carefully selected patients can be considered once stabilization is achieved, with some suggestion that oral antibiotics may be an option.Evidence 3Low Quality of Evidence (low confidence that we know true effects of the intervention). Quality of Evidence lowered due to indirectness. Quality of Evidence Lowered due to imprecision and indirectness. Iversen K, Ihlemann N, Gill SU, et al. Partial Oral versus Intravenous Antibiotic Treatment of Endocarditis. N Engl J Med. 2019 Jan 31;380(5):415-424. doi: 10.1056/NEJMoa1808312. Epub 2018 Aug 28. PMID: 30152252.
Treatment may vary in duration from 2 weeks (in a highly selective population meeting predefined criteria) up to 4 to 6 weeks (most commonly) or longer.
Treatment of PVE lasts ≥6 weeks but is otherwise similar.
1. IV antibiotics: In acutely ill patients with suspected IE, start empiric treatment immediately after obtaining blood cultures (Figure 3.10-5; also see Table 10 in ESC guidelines). As indicated above, the choice of empiric treatment should take into consideration patient characteristics, previous antimicrobial therapy, epidemiological features, and the most likely pathogens. It should also involve an infectious disease specialist if feasible.
There is only limited and low-quality evidence on the comparative effects of different antibiotic regimens with some regional preferences for therapy. Whenever applicable, treatment should be tailored to blood culture results (identified pathogens) and sensitivities once these become available; for instance, streptococcal IE: Figure 3.10-6 (also see Table 7 in ESC guidelines); staphylococcal IE: Figure 3.10-7 (also see Table 8 in ESC guidelines); IE caused by other pathogens (including those causing IE with negative blood cultures): Table 3.10-2 (also see Tables 9 and 11 in ESC guidelines). There may be differences between different practice guidelines. For example, the European guidelines in some situations list amoxicillin, whereas the AHA guidelines list ampicillin.
2. Antithrombotic treatment: Data to guide the use of antithrombotic therapy in the setting of IE are limited, and decisions should be made on a case-by-case basis with consideration given to risks and benefits. IE by itself is not an indication for antithrombotic treatment, but such therapy is generally continued if started previously for other indications in the absence of contraindications. In patients who may need to be qualified for urgent surgery or with international normalized ratio (INR) values showing wide variations, anticoagulation is often switched to unfractionated heparin (UFH). For patients with IE and stroke, thrombolytic therapy is not recommended. However, thrombectomy may be considered in selected cases with large vessel occlusion. In cases of ischemic or hemorrhagic stroke, all anticoagulants and antiplatelet drugs should be stopped for a minimum of 2 weeks. The optimal length of time for discontinuation is unknown and based on limited evidence. In patients with a prosthetic heart valve and intracerebral hemorrhage, antithrombotic treatment (using UFH) should be resumed as soon as it is safe to do so. In the event of serious hemorrhagic complications, antithrombotic treatment should be stopped, and neurology and hematology service consultations are prudent.
The need for and the optimal timing of surgical treatment are among the most difficult therapeutic decisions in the treatment of IE. Surgical intervention may be prompted by hemodynamic instability despite antimicrobial therapy. Decisions regarding surgery should be made by an experienced clinical team with cardiac surgery service and infectious disease consultations and should consider individual risks and benefits. Preoperative computed tomography angiography (CTA) should be used to assess coronary anatomy and coronary artery disease prior to surgical intervention.
Consider emergency surgery (within the first 24 hours): Refractory pulmonary edema or cardiogenic shock caused by severe acute mitral or aortic regurgitation, obstruction, intracranial hemorrhage, or fistula.Evidence 4 Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of the intervention). Nadji G, Rusinaru D, Rémadi JP, Jeu A, Sorel C, Tribouilloy C. Heart failure in left-sided native valve infective endocarditis: characteristics, prognosis, and results of surgical treatment. Eur J Heart Fail. 2009 Jul;11(7):668-75. doi: 10.1093/eurjhf/hfp077. PubMed PMID: 19553397. Thuny F, Beurtheret S, Mancini J, et al. The timing of surgery influences mortality and morbidity in adults with severe complicated infective endocarditis: a propensity analysis. Eur Heart J. 2011 Aug;32(16):2027-33. doi: 10.1093/eurheartj/ehp089. Epub 2009 Mar 26. PubMed PMID: 19329497. Kiefer T, Park L, Tribouilloy C, et al. Association between valvular surgery and mortality among patients with infective endocarditis complicated by heart failure. JAMA. 2011 Nov 23;306(20):2239-47. doi: 10.1001/jama.2011.1701. PubMed PMID: 22110106; PubMed Central PMCID: PMC5030065.
Consider urgent surgery in active IE:
1) Symptoms of heart failure or echocardiographic signs of poor hemodynamic tolerance caused by severe regurgitation or obstruction of a native or prosthetic valve.
2) Locally uncontrolled infection with involvement of perivalvular structures (annular or aortic abscess, fistula, leaflet rupture, conduction disturbances).
3) Persistent infection despite appropriate antibiotic treatment: This is variably defined as persisting bacteremia and fever after >5 to 7 days and exclusion of other causes of persistent positive blood cultures.
4) Infection with difficult-to-treat organisms (fungi or multidrug-resistant organisms).
5) PVE endocarditis caused by staphylococci or non-HACEK gram-negative bacteria.
6) Persistent aortic or mitral vegetations >10 mm after ≥1 embolic episode despite appropriate antibiotic treatment (particularly during the first 2 weeks of treatment).
7) Aortic or mitral NVE vegetations >10 mm associated with severe valve stenosis or regurgitation and with low operative risk.
8) Aortic or mitral NVE and PVE with isolated large vegetations (>15 mm) and no other indications for surgery.
9) Aortic or mitral NVE and PVE with isolated very large vegetations (>30 mm).
Decision for surgery in patients with large vegetations (>15 mm) can be difficult and requires a careful individual approach.
Surgical treatment may also be considered after CNS embolism (including transient ischemic attack or asymptomatic embolism) as long as intracranial hemorrhage has been excluded, the patient is not in a comatose state, stroke has not caused very severe CNS damage, and no other contraindications to surgery are present. The risk of an embolic event is highest before the start of antimicrobial therapy and in the early days of treatment and decreases significantly after 2 weeks of therapy. Head CT or MRI should be conducted in every patient with suspected neurologic complications. Indications for surgery in such patients include severe heart failure, uncontrolled sepsis, infection not responding to antimicrobial therapy, presence of an abscess, or persistence of a high embolic risk. Surgery using a cardiopulmonary bypass within the first 30 days of stroke is associated with a high risk of complications.
Treatment of CDRIE: Prolonged antimicrobial treatment (initially empiric, optimally using an antistaphylococcal agent [vancomycin, given the high resistance rates], followed by targeted treatment based on susceptibility testing) and complete hardware removal is generally recommended.Evidence 5 Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). Low Quality of Evidence (low confidence that we know true effects of the intervention). Quality of Evidence lowered due to the observational nature of data. Le KY, Sohail MR, Friedman PA, et al; Mayo Cardiovascular Infections Study Group. Impact of timing of device removal on mortality in patients with cardiovascular implantable electronic device infections. Heart Rhythm. 2011 Nov;8(11):1678-85. doi: 10.1016/j.hrthm.2011.05.015. Epub 2011 May 27. PubMed PMID: 21699855. A center capable of performing percutaneous removal of the implanted device including leads should be involved in such cases. In patients with vegetations >20 mm surgical treatment should be considered. Given the risk of reinfection, indications for reimplantation of devices should be evaluated. If the patient is clinically stable, blood cultures should be negative for a minimum of 72 hours while antimicrobial therapy is continued. If the patient is dependent on the device, a temporary electrode should be implanted contralaterally. In case of evidence of remaining valvular infection, implantation should be delayed for 14 days.
Treatment of IE in IV drug users: Valve replacement is rarely recommended because substance dependence results in frequent recurrences of IE in this group of patients. Surgery may be considered in those with bacteremia persisting >7 days in spite of adequate antimicrobial treatment, fungal infections, right ventricular heart failure refractory to treatment, or those with persisting large (>20 mm) vegetations with recurrent pulmonary embolism.
The duration of therapy varies based on operative tissue cultures when surgery is pursued. In patients with negative operative tissue cultures, the first day of negative blood cultures on antimicrobial treatment is the first day from which the duration of targeted therapy is calculated. In patients with positive valve tissue cultures, a new postoperative complete course of antimicrobial treatment using agents to which the isolated organisms are susceptible is recommended (with the operative day considered as day 0).
Follow-UpTop
The patient should be carefully monitored for cardiac and extracardiac complications, especially for embolic events. In particular, the following parameters should be monitored: temperature (febrile or afebrile), white blood cell counts, and biochemical markers associated with any potential drug toxicities (eg, renal function). Repeat blood cultures after 48 to 72 hours should be collected to evaluate treatment response, with additional repeats every 1 to 3 days until clearance of bacteremia is documented. Serial physical examinations should also be conducted to evaluate for endocarditis-related complications and sequelae. In patients undergoing antimicrobial treatment the severity of valvular disease and any indications for surgery should be evaluated. After completing treatment, perform follow-up TTE for functional and morphologic evaluation.
Regular clinical follow-up is recommended following the completion of initial therapy.
ComplicationsTop
PreventionTop
1. General preventative measures are important, such as maintenance of general oral and skin hygiene, avoiding body piercings and tattoos, and minimizing unnecessary IV catheterization and invasive procedures.
2. Indications for antimicrobial prophylaxis: Indications for IE prophylaxis have been narrowed over time. Perioperative antibiotic prophylaxis is recommended in all patients undergoing implantation of a prosthetic valve, any type of prosthetic graft/occluder device (patent foramen ovale or patent ductus arteriosus occluding devices) or CIED. In all other cases prophylaxis is currently indicated only before dental procedures involving gingival or periapical instrumentation or perforation of the oral mucosa (dental extraction, periodontal procedures, root canal treatment, scaling, dental implants)Evidence 6 Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due to indirectness. Cahill TJ, Harrison JL, Jewell P, et al. Antibiotic prophylaxis for infective endocarditis: a systematic review and meta-analysis. Heart. 2017 Jun;103(12):937-944. doi: 10.1136/heartjnl-2015-309102. Epub 2017 Feb 17. Review. PubMed PMID: 28213367. and only in the following specific circumstances:
1) Prosthetic valve (including transcatheter valve replacement) or history of valve repair using prosthetic materials.
2) History of IE.
3) Congenital heart disease (cyanotic; up to 6 months after complete surgical or percutaneous repair of congenital heart disease using prosthetic materials; residual regurgitation or leak in the area of surgical or transcatheter implantation of prosthetic material).
4) Cardiac transplant patients with a structurally abnormal valve (as per the AHA/American Society of Cardiology recommendations).
5) Ventricular assist devices (VADs).
3. Recommended antimicrobial agents (a single dose 30-60 minutes before the procedure):
1) Patients with no allergy to penicillin: Oral or IV amoxicillin or ampicillin 2 g in adults or 50 mg/kg in children. Alternative agents are IV cefazolin or ceftriaxone 1 g in adults or 50 mg/kg in children.
2) Patients with allergy to penicillin: Oral cephalexin 2 g in adults or 50 mg/kg in children (this should not be used in an individual with a history of anaphylaxis, angioedema, or urticaria with penicillin or ampicillin), or IV cefazolin 1 g in adults or 50 mg/kg in children.
Tables and FiguresTop
|
Pathologic criteria |
|
1) Microorganisms demonstrated by culture or on histologic examination of a vegetation, a vegetation that has embolized, or an intracardiac abscess specimen; or 2) Pathologic lesions; vegetation or intracardiac abscess confirmed by histologic examination showing active endocarditis. |
|
Clinical criteria |
|
Major criteria 1) Blood cultures positive for IE: a) Typical microorganisms consistent with IE from 2 separate blood cultures: viridans group streptococci, Streptococcus gallolyticus (formerly S bovis), HACEK group, Staphylococcus aureus, Enterococcus faecalis; or b) Microorganisms consistent with IE from persistent positive blood cultures: ≥2 positive blood cultures of samples drawn >12 h apart; or all of 3 or a majority of ≥4 separate blood cultures (first and last drawn ≥1 h apart); or c) Single positive blood culture for Coxiella burnetii or phase I IgG antibody titer >1:800. 2) Imaging studies positive for IE: Valvular, perivalvular/periprosthetic and foreign material anatomic and metabolic lesions characteristic of IE detected by any of the following imaging techniques: a) Echocardiography (TTE and TEE). b) Cardiac CT. c) FDG-PET/CT(A). d) WBC SPECT/CT. Minor criteria 1) Predisposition, eg, predisposing heart condition or IV drug use. 2) Fever (defined as >38 degrees Celsius). 3) Vascular phenomena (including those detected by imaging only): Major arterial emboli, septic pulmonary infarcts, hematogenous osteoarticular septic complications (spondylodiscitis), infectious (mycotic) aneurysm, intracranial hemorrhage, conjunctival hemorrhages, Janeway lesions. 4) Immunologic phenomena: Glomerulonephritis, Osler nodes and Roth spots, rheumatoid factor. 5) Microbiologic evidence: Positive blood culture but not meeting major criteria (above) or serologic evidence of active infection with organism consistent with IE. |
|
Definite IE: 2 major criteria; 1 major criterion and 3 minor criteria; or 5 minor criteria. Possible IE: 1 major criterion and 1 or 2 minor criteria; or 3-4 minor criteria. Rejected IE: 1) Firm alternative diagnosis; or 2) Resolution of symptoms suggesting IE with antibiotic therapy for ≤4 days; or 3) No pathologic evidence of IE at surgery or autopsy with antibiotic therapy for ≤4 days; or 4) Not fulfilling the criteria for possible IE, as defined above. |
|
Based on Eur Heart J. 2023;44(39):3948-4042. |
|
CT, computed tomography; CT(A), computed tomography (angiography); FDG-PET, 18F-fluorodeoxyglucose positron emission tomography; HACEK, Haemophilus spp, Aggregatibacter spp, Cardiobacterium hominis, Eikenella corrodens, Kingella spp; IE, infective endocarditis; IV, intravenous; SPECT, single-photon emission tomography; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography; WBC, white blood cell. |
|
Enterococci |
|
– Amoxicillin (or ampicillin) 200 mg/kg/d IV in 4-6 divided doses for 4-6 weeksa + gentamicinb 3 mg/kg/d IV or IM as single dose for 2-6 weeks; or – Ampicillin 200 mg/kg/d IV in 4-6 divided doses for 6 weeks + ceftriaxone 4 g/d IV or IM in 2 divided doses for 6 weeksc; or – Vancomycin 30 mg/kg/d in 2 divided doses for 6 weeks + gentamicinb 3 mg/kg/d IV as single dose for 6 weeks |
|
Beta-lactam–resistant strains of enterococci – Resistance due to beta-lactamase production: Regimens with ampicillin/sulbactam or amoxicillin/clavulanic acid in place of ampicillin or amoxicillin – Resistance caused by penicillin-binding proteins: Vancomycin regimen |
|
Enterococci with high levels of aminoglycoside resistance Ampicillin or amoxicillin + ceftriaxone for 6 weeks |
|
Vancomycin-resistant enterococci Daptomycin 10-12 mg/kg/d combined with one of: ampicillin (300 mg/kg/d), ertapenem (2 g/d in single dose), ceftaroline (1800 mg/d in 3 doses), or fosfomycin (12 g/d in 4 doses) for 6 weeks |
|
Multidrug-resistant strains (resistance to aminoglycosides, beta-lactams, and vancomycin)d – Daptomycin 10 mg/kg/d combined with one of: ampicillin 200 mg/kg/d IV in 4-6 doses for ≥8 weeks; ertapenem (2 g/d IV), ceftaroline (600 mg tid IV), or fosfomycin (3 g IV qid); or – Linezolid 600 mg IV or PO bid for ≥8 weeks; or – Quinupristin/dalfopristin 7.5 mg/kg tid for ≥8 weeks |
|
HACEK group |
|
Third-generation cephalosporin (eg, ceftriaxone 2 g/d for 4 weeks in NVE or 6 weeks in PVE); non–beta-lactamase-producing strains: ampicillin 12 g/d IV in 4 or 6 divided doses combined with gentamicin 3 mg/kg/d in 2 or 3 divided doses for 4-6 weeks |
|
Brucella spp |
|
Doxycycline 200 mg/d + trimethoprim/sulfamethoxazole 960 mg bid + rifampin 300-600 mg/d for ≥3-6 months PO; in the first few weeks you may add streptomycin 15 mg/kg/d in 2 divided doses |
|
Coxiella burnetii |
|
Doxycycline 200 mg/d + hydroxychloroquine 200-600 mg/d PO (this is preferred over doxycycline alone) for >18 months |
|
Bartonella spp |
|
Doxycycline 100 mg PO bid for 4 weeks + gentamicin 3 mg/kg/d IV for 2 weeks |
|
Legionella spp |
|
Levofloxacin 500 mg IV or PO bid for ≥6 weeks or clarithromycin 500 mg bid IV for 2 weeks, then PO for 4 weeks + rifampin 300-1200 mg for 6 weeks |
|
Mycoplasma spp |
|
Levofloxacin 500 mg IV or PO bid for ≥6 months |
|
Tropheryma whipplei |
|
Doxycycline 200 mg/d + hydroxychloroquined 200-600 mg/d PO (this is preferred over doxycycline alone) for ≥18 months |
|
a Treatment lasting 6 weeks is recommended in patients with symptoms persisting for >3 months and in those with a prosthetic valve. 2015 AHA guidelines allow aqueous penicillin G 18-30 million units/d in place of ampicillin. c Regimen recommended in Enterococcus faecalis infection. It is the treatment of choice for aminoglycoside-resistant E faecalis species. Ineffective for Enterococcus faecium. d Cooperation with an infectious disease specialist is essential. |
|
Based on Eur Heart J. 2023;44(39):3948-4042. |
|
bid, 2 times a day; HACEK, Haemophilus spp, Aggregatibacter spp, Cardiobacterium hominis, Eikenella corrodens, Kingella spp; IM, intramuscular; IV, intravenous; NVE, native valve endocarditis; PO, oral; qid; 4 times a day; PVE, prosthetic valve endocarditis; tid, 3 times a day. |
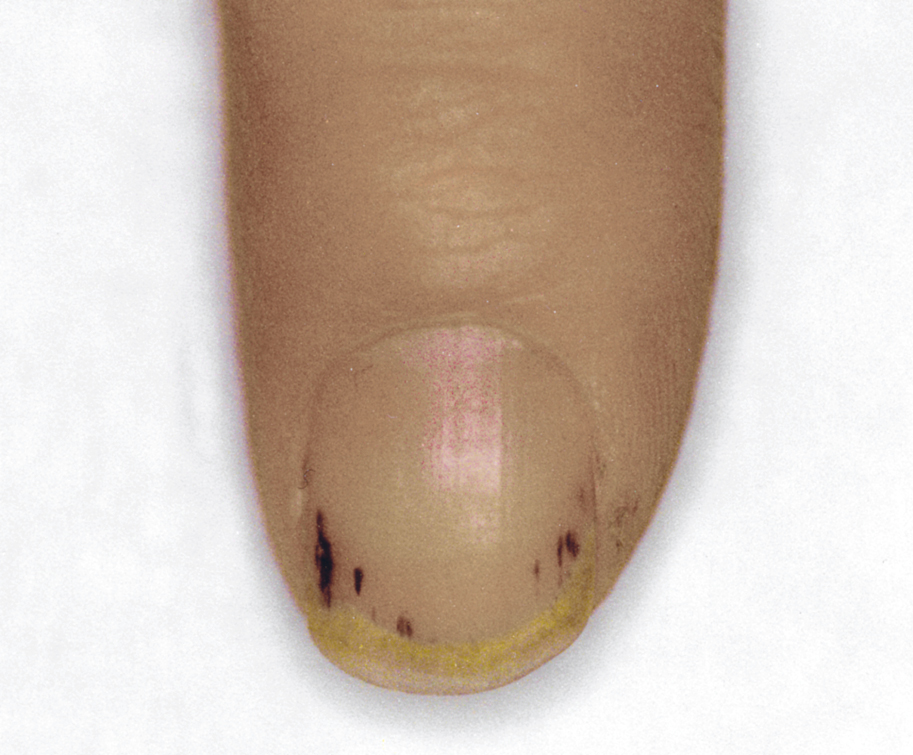
Figure 3.10-1. Petechiae under nail plates in a patient with infective endocarditis.
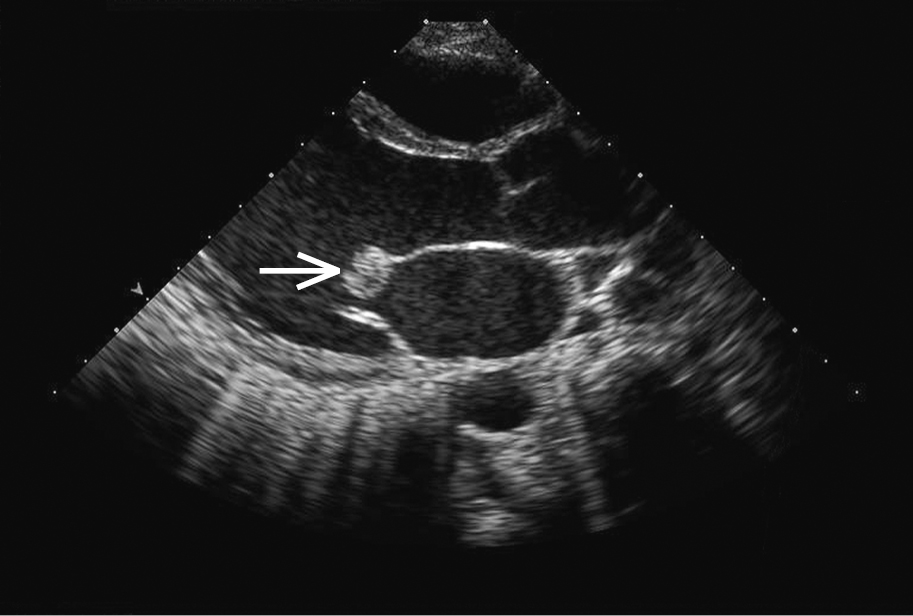
Figure 3.10-2. Transthoracic echocardiography (TTE): a large mobile vegetation (arrow) on the anterior mitral leaflet in a patient with infective endocarditis. Figure courtesy of Dr Marek Krzanowski.
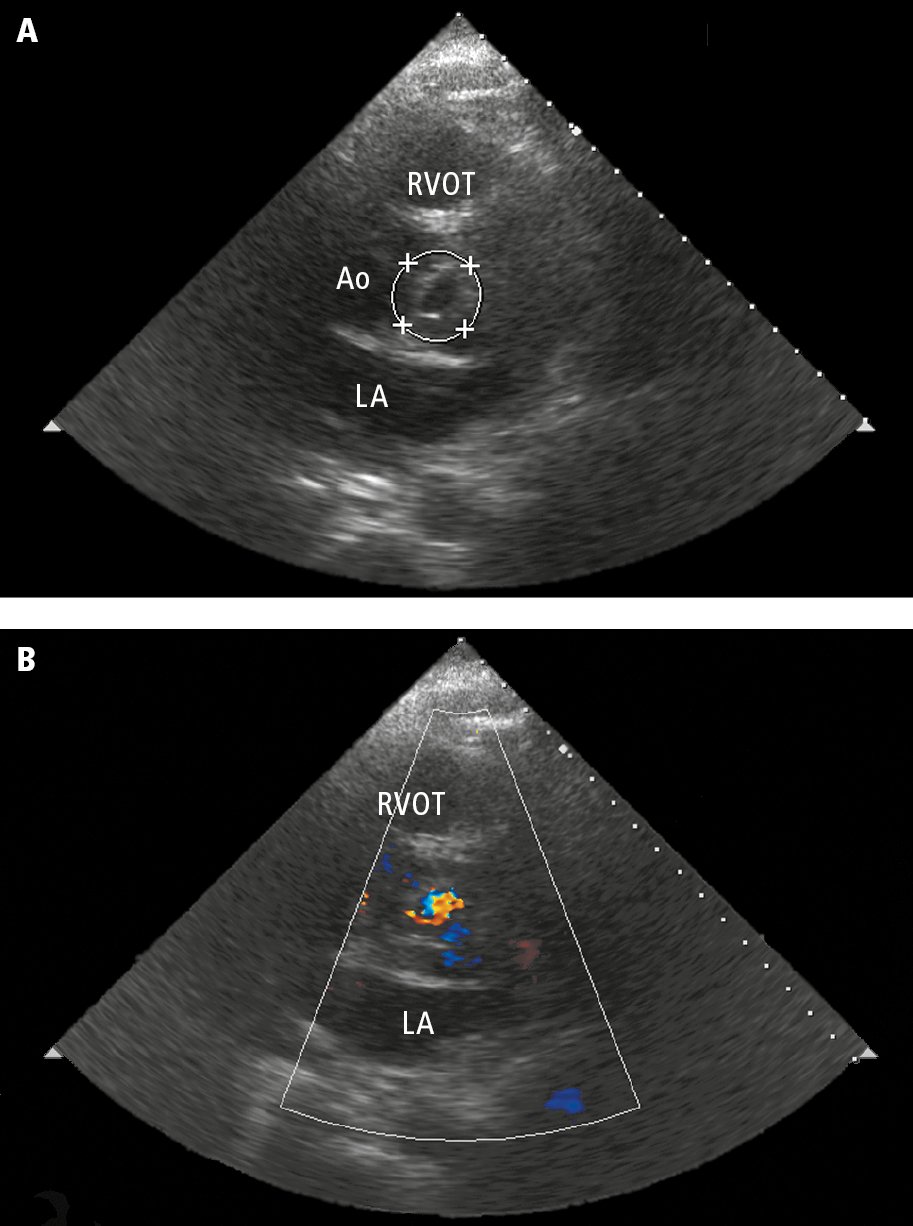
Figure 3.10-3. Transthoracic echocardiography (TTE): the parasternal short-axis view at the level of the aortic valve. A, left coronary leaflet perforation (circle). B, color Doppler ultrasonography showing a jet of aortic regurgitation through the perforated leaflet. Ao, aorta; LA, left atrium; RVOT, right ventricular outflow tract.
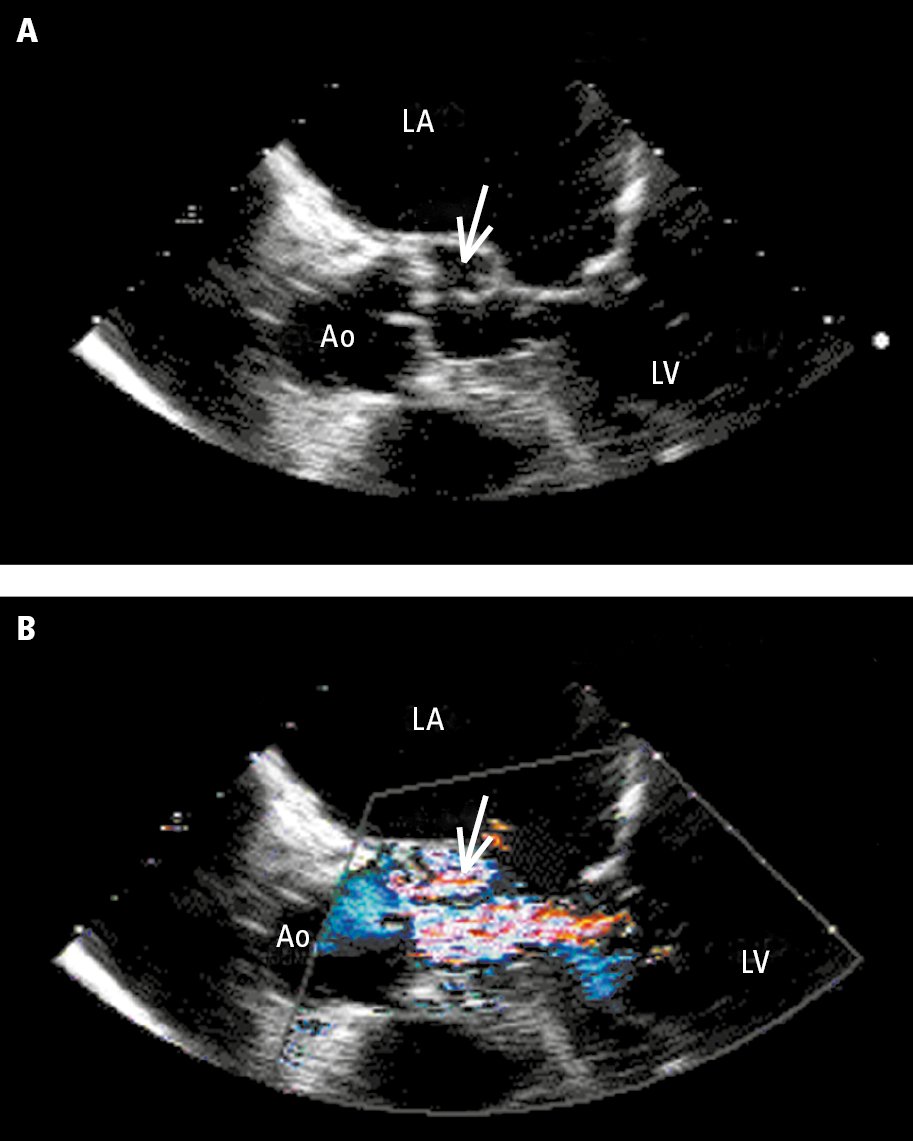
Figure 3.10-4. Transesophageal echocardiography (TEE): A, aortic valve (Ao) with a periaortic abscess (arrow); B, color Doppler ultrasonography showing a jet of aortic valve regurgitation flowing into the abscess. Ao, aorta; LA, left atrium; LV, left ventricle. Figure courtesy of Dr Marek Krzanowski.
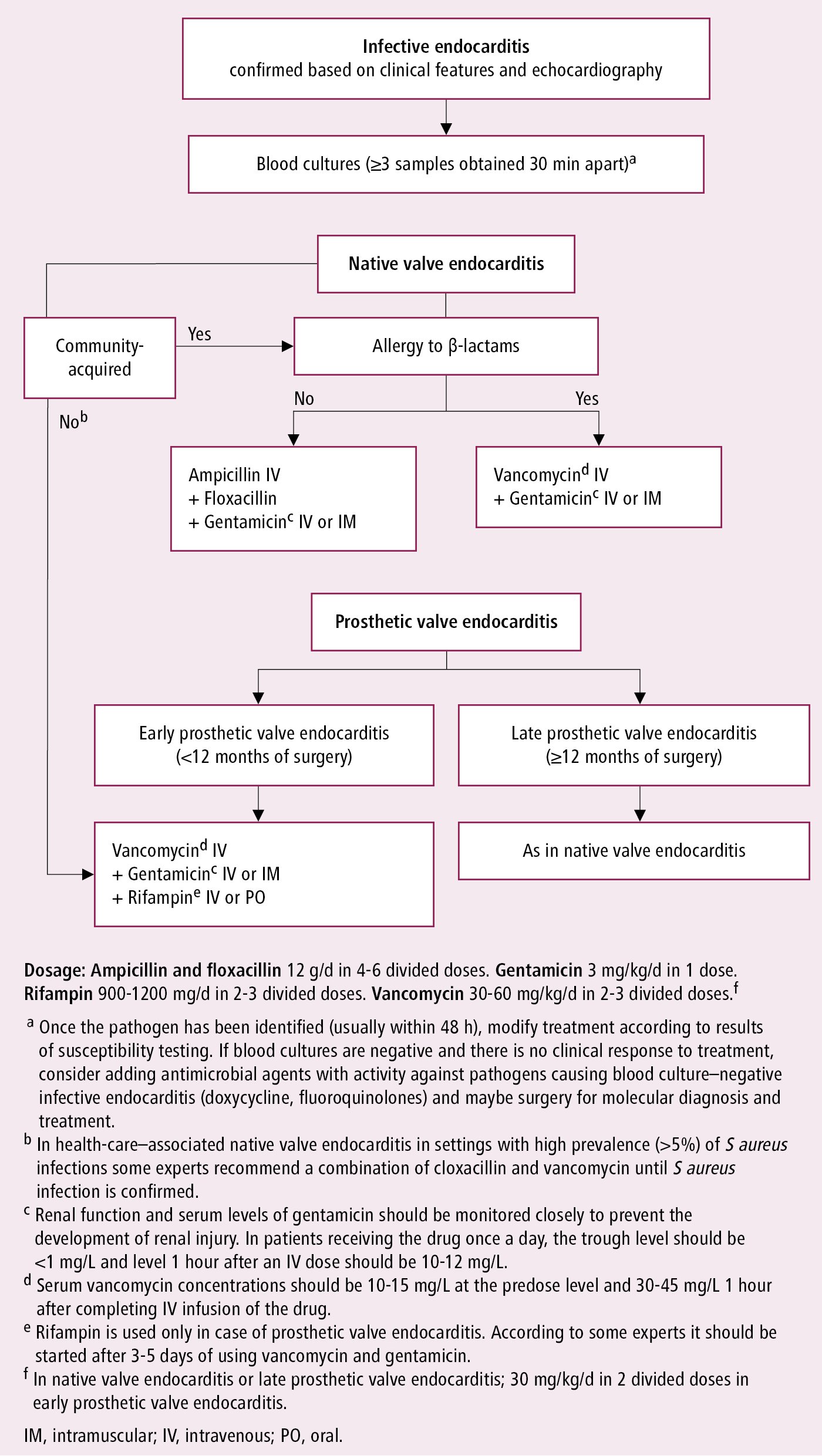
Figure 3.10-5. Empiric antibiotic treatment for infective endocarditis before pathogen identification and in the case of negative cultures. Based on Eur Heart J. 2023;44(39):3948-4042.
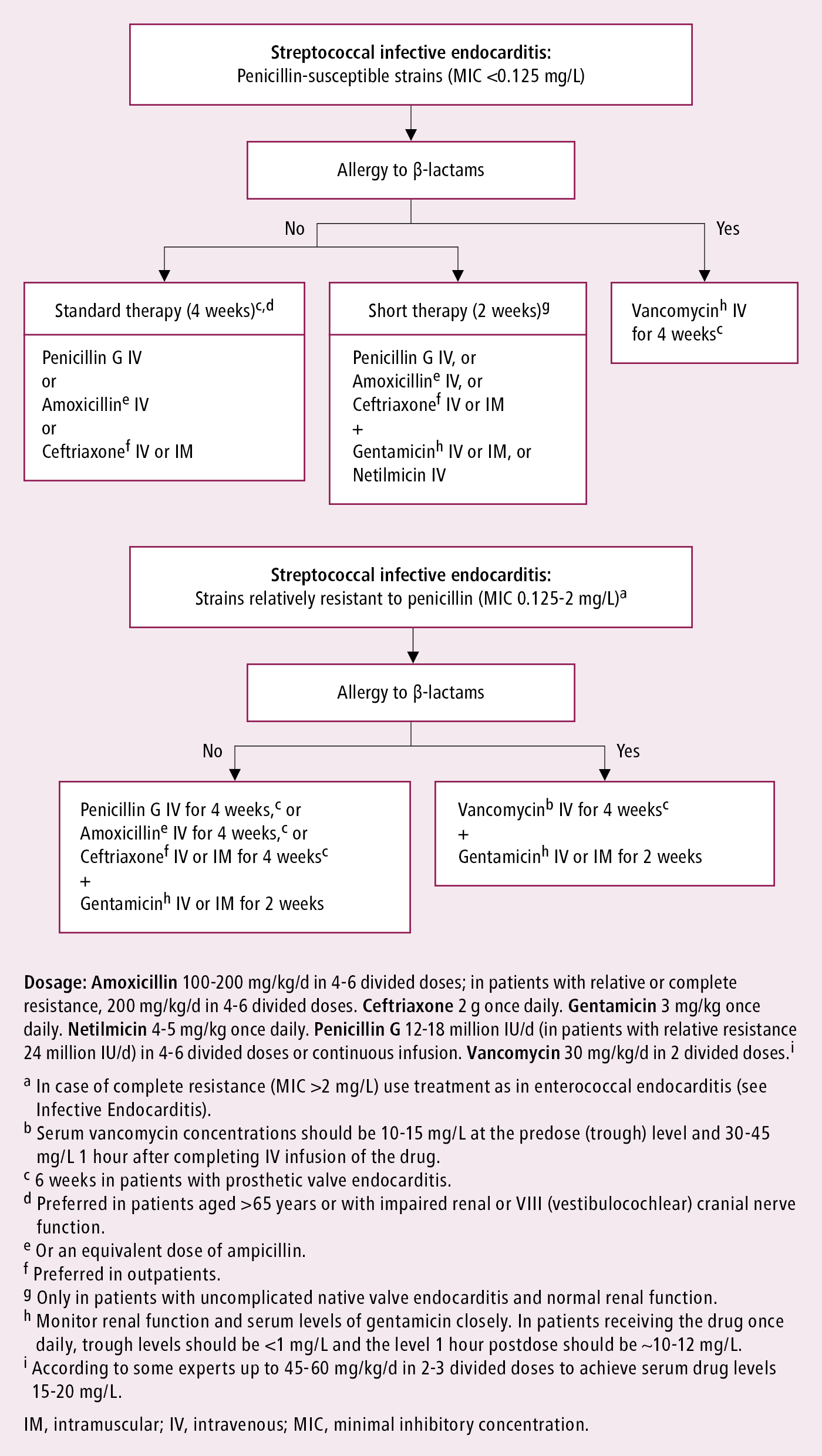
Figure 3.10-6. Infective endocarditis caused by oral streptococci or Streptococcus gallolyticus group: targeted antibiotic treatment. Based on Eur Heart J. 2023;44(39):3948-4042.
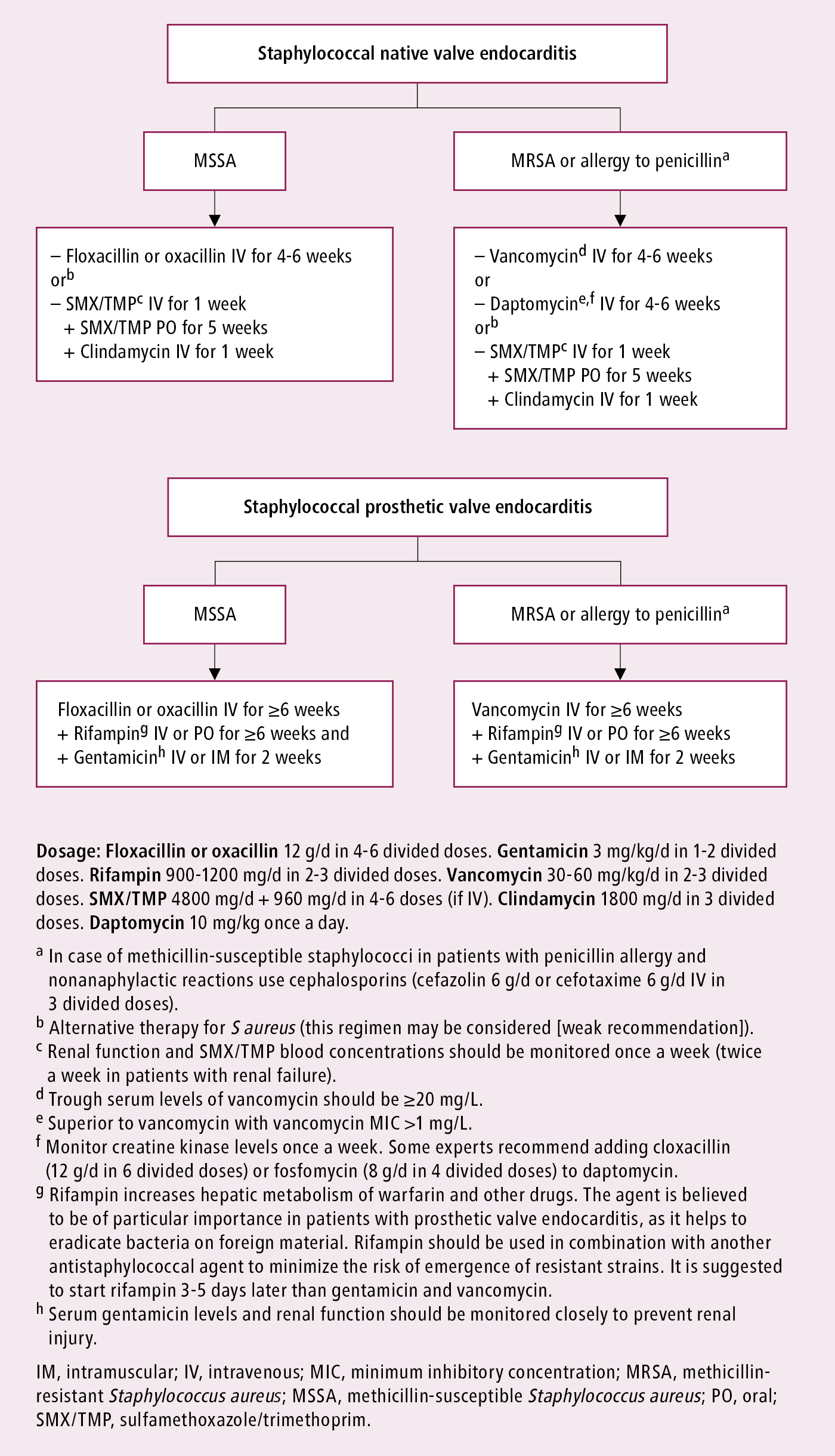
Figure 3.10-7. Infective endocarditis caused by staphylococci: targeted antibiotic treatment. Based on Eur Heart J. 2023;44(39):3948-4042.