Primary Panel:, Abramson BL, Al-Omran M, et al. Canadian Cardiovascular Society 2022 Guidelines for Peripheral Arterial Disease. Can J Cardiol. 2022;38(5):560-587. doi:10.1016/j.cjca.2022.02.029
Aboyans V, Ricco JB, Bartelink MEL, et al; ESC Scientific Document Group. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. Endorsed by: the European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J. 2018 Mar 1;39(9):763-816. doi: 10.1093/eurheartj/ehx095. PubMed PMID: 28886620.
European Stroke Organisation, Tendera M, Aboyans V, Bartelink ML, et al; ESC Committee for Practice Guidelines.ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2011 Nov;32(22):2851-906. doi: 10.1093/eurheartj/ehr211. Epub 2011 Aug 26. PubMed PMID: 21873417.
Rooke TW, Hirsch AT, Misra S, et al; Society for Cardiovascular Angiography and Interventions; Society of Interventional Radiology; Society for Vascular Medicine; Society for Vascular Surgery. 2011 ACCF/AHA Focused Update of the Guideline for the Management of Patients With Peripheral Artery Disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011 Nov 1;58(19):2020-45. doi: 10.1016/j.jacc.2011.08.023. Epub 2011 Oct 6. PubMed PMID: 21963765; PubMed Central PMCID: PMC4714326.
Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Eur J Vasc Endovasc Surg. 2007;33 Suppl 1:S1-75. Epub 2006 Nov 29. PubMed PMID: 17140820.
Definition, Etiology, PathogenesisTop
Lower extremity peripheral artery disease (PAD) is a condition in which the supply of oxygen to tissues of the lower extremities is inadequate due to chronically impaired arterial blood flow. In >97% of patients this is caused by atherosclerosis of the lower extremities. Other causes of chronic lower extremity ischemia: see Differential Diagnosis, below.
Clinical FeaturesTop
The staging of PAD is based on the severity of symptoms: Table 3.19-5.
PAD is often accompanied by features of atherosclerosis of other arteries: coronary, cranial, or renal. Sometimes patients have a coexisting abdominal aortic aneurysm.
1. Symptoms: Early PAD is asymptomatic. With time, patients develop easy fatigability of the limbs, increased sensitivity to cold, and paresthesia. They usually seek medical attention because of intermittent claudication, that is, pain occurring relatively regularly after defined physical exercise (eg, walking a certain distance). The pain, sometimes described as muscle numbness or stiffness, involves muscles below the level of artery stenosis/occlusion, is not referred, forces the patient to stop walking, and resolves spontaneously after several seconds or minutes of rest. Claudication pain usually involves large muscle groups of the lower extremity, such as the buttocks, thighs, and most frequently the calf. Claudication of the foot (deep pain in the middle of the foot [short flexor muscles of the foot]) occurs rarely and results from narrowing or occlusion of the more distal arteries in the lower leg (tibial arteries) or foot. It can be seen in conditions such as thromboangiitis obliterans (Buerger disease) and diabetes mellitus. Severe narrowing or occlusion of the aorta, iliac arteries, or both may present as Leriche syndrome, which is defined as intermittent claudication, absent femoral pulses, and erectile dysfunction.
In advanced stages patients complain of foot or leg pain (or both) occurring at night (often hanging the limb over the side of a bed to keep it in the lowest possible position and allow gravity to improve blood flow) or pain at rest throughout the day and night.
2. Signs: The skin of the feet is pale (Figure 3.19-1) or cyanotic (especially when standing) and cold. In advanced PAD, trophic lesions (discoloration, hair loss, ulcers, necrosis [Figure 3.19-2]) may be present. Patients also have muscle atrophy and a weak, absent, or asymmetric pulse over the arteries below the stenosis/occlusion, sometimes with a vascular bruit over the large arteries of the extremities. On the lower extremities, pulse is assessed on the following arteries: the dorsalis pedis artery (on the dorsum of the foot between the first and second metatarsal bone; not palpable in 8% of healthy persons), posterior tibial artery (behind the medial malleolus), popliteal artery (in the popliteal fossa), and femoral artery (in the groin, just below the inguinal ligament).
DiagnosisTop
1. Ankle-brachial index (ABI) (Figure 3.19-3): A ratio of the systolic blood pressure (SBP) measured using continuous wave Doppler ultrasonography in the ankle to the SBP measured in the arm (if pressures vary between the arms, consider the higher value). A normal value is from 0.9 to 1.15; ABI <0.9 indicates the presence of stenosis (in critical ischemia usually <0.5), while ABI >1.3 is suggestive of abnormal arterial stiffness (eg, in patients with diabetes mellitus).
If tibial arteries cannot be compressed due to their stiffness, use the toe-brachial index (TBI) (Figure 3.19-4). The measuring principle is the same as in the ABI, but SBP is measured in the toe; the pressure measured in the toe should be ~10 mm Hg lower than the pressure measured at the level of the ankle. A normal TBI is >0.7; lower values indicate the possibility of lower limb ischemia.
2. Stress testing on a treadmill is performed in case of diagnostic doubts, particularly in patients with borderline ABI values, as well as to objectively assess the claudication distance. The ABI is calculated before and at the peak of exercise. If pain forcing the patient to interrupt testing is caused by ischemia, the ankle pressure after exercise will be significantly lower than before exercise (often <50 mm Hg).
3. Imaging studies: Arterial duplex ultrasonography of the arteries (including B-mode imaging [ultrasonography] and Doppler spectral waveform analysis) is the key method used in the initial diagnostics and for surveillance of patients with PAD, in particular those who have undergone surgical treatment (patency of prosthesis and bypasses) or endovascular interventions. The study should always be preceded by a thorough physical examination and ABI measurement. Computed tomography angiography (CTA) and magnetic resonance angiography (MRA) allow for the assessment of the entire vascular system and type of lesions in the vascular walls as well as qualification or planning of the patient for an appropriate invasive procedure; these studies should not be used for screening. Arteriography is performed in the case of diagnostic doubts or more commonly in the course of therapeutic endovascular procedures such as angioplasty (with or without stenting).
The diagnosis of PAD is made on the basis of symptoms, signs, and ABI (or the walking test). If arteries at the ankle cannot be compressed or if the ABI is >1.40, use alternative methods (eg, TBI or Doppler). Critical limb ischemia is diagnosed in a patient with chronic ischemia with pain at rest, necrosis, or ulceration (Fontaine classification stages III-IV or Rutherford stages 4-6).
1. Causes of chronic lower limb ischemia other than atherosclerosis: There are multiple other nonatherosclerotic causes of lower extremity arterial narrowing or occlusion that can present as chronic lower limb ischemia. Peripheral emboli from the heart (arrhythmias, valvular heart disease) or proximal arteries (plaque or aneurysms) may more typically present as acute ischemia but can also cause chronic symptoms. Inflammatory changes as well as thickening and narrowing of the artery wall and lumen can occur with large-vessel vasculitis, thromboangiitis obliterans (Buerger disease), and radiation injury (especially in iliac arteries following pelvic irradiation). Trauma can narrow arteries with dissection or chronic repetitive injury including external iliac artery endofibrosis (cyclist hip pain). The popliteal artery in particular can be extrinsically narrowed by muscles and tendons (popliteal artery entrapment) or adventitial cysts. Other rare congenital causes include coarctation of the aorta, fibrous dysplasia, and anomalous development of the lower extremity vasculature, such as arteriovenous malformations and persistent sciatic artery.
2. Differential diagnosis of intermittent claudication: There are many diseases or conditions (most often musculoskeletal or neurogenic) that can be confused with vascular claudication. Common conditions that may need to be differentiated from vascular discomfort: Table 3.19-6.
TreatmentTop
1. The treatment strategy is established on an individual basis depending on the severity of disease as well as the patient’s general condition, age, activity level (including type of occupation), and comorbidities.
2. Management includes (1) secondary prevention of cardiovascular diseases; (2) treatment aimed at improving walking distance or in more advanced cases at alleviating night or rest pain and avoiding major limb loss (amputation). These treatments may be nonpharmacologic, pharmacologic, or invasive.
1. Lifestyle changes for secondary prevention of cardiovascular diseases. Smoking cessation is of key importance.
2. Regular walking exercises: These are used to increase the claudication distance. Walk intensity should be adapted to the symptoms (it should not cause pain). We suggest that our patients walk at a slow to moderate pace until they feel some discomfort. Some suggest regular walking or cycling. Beneficial effects decrease or disappear with the discontinuation of regular exercise.
1. To prevent cardiovascular incidents, every patient should receive long-term treatment with an antiplatelet agent. Use acetylsalicylic acid (ASA) 75 to 150 mg/d; if this is contraindicated, use clopidogrel 75 mg/d or ticlopidine 250 mg bid. Use a statin, which may additionally help increase the claudication distance.
2. A combination of low-dose rivaroxaban (2.5 mg bid) with low-dose ASA (100 mg once a day) is an option.Evidence 1Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). High Quality of Evidence (high confidence that we know true effects of the intervention). Anand SS, Bosch J, Eikelboom JW, et al; COMPASS Investigators. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet. 2018 Jan 20;391(10117):219-229. doi: 10.1016/S0140-6736(17)32409-1. Epub 2017 Nov 10. PubMed PMID: 29132880.
3. The effectiveness of cilostazol or nafronyl oxalate (INN naftidrofuryl) in increasing the claudication distance is low. The evidence on the effectiveness of pentoxifylline is not convincing.
1. Indications: Critical lower limb ischemia (Fontaine stages III-IV, Rutherford stages 4-6 [Table 3.19-5]). Patients without critical limb ischemia but with moderate to severe claudication (Fontaine IIb, Rutherford 2-3) that is not improved by medical management and interferes with work activities or quality of life are often considered for invasive treatment if they have a good procedural risk and are determined to have anatomy favorable for successful intervention.
2. Treatment techniques: Percutaneous endovascular treatment (with or without stent implantation), surgical treatment (bypass graft implantation, endarterectomy, or both). In patients with extensive tissue loss (Rutherford stage 6), consideration may be given to proceed to primary amputation without revascularization.
3. After surgery: After implantation of a bare-metal stent below the groin, dual antiplatelet therapy (ASA + clopidogrel) for ≥1 month is recommended. In patients at increased risk of thrombosis in the bypass (eg, history of thrombotic events, polycythemia), chronic anticoagulant treatment with a vitamin K antagonist (acenocoumarol or warfarin) may be of value. The evidence is evolving and recent data suggest benefit of long-term low-dose ASA combined with low-dose rivaroxaban.Evidence 2High Quality of Evidence (high confidence that we know true effects of the intervention). Eikelboom JW, Connolly SJ, Bosch J, et al; COMPASS Investigators. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N Engl J Med. 2017 Oct 5;377(14):1319-1330. doi: 10.1056/NEJMoa1709118. Epub 2017 Aug 27. PubMed PMID: 28844192. Anand SS, Bosch J, Eikelboom JW, et al; COMPASS Investigators. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet. 2018 Jan 20;391(10117):219-229. doi: 10.1016/S0140-6736(17)32409-1. Epub 2017 Nov 10. PubMed PMID: 29132880. Bonaca MP, Bauersachs MD, Anand SS, et al. Rivaroxaban in Peripheral Arterial Disease after Revascularization. N Engl J Med. 2020 May 21;382(21):1994-2004. doi: 10.1056/NEJMoa2000052. PMID: 32222135. Clinical follow-up and ultrasonography could be performed 1, 3, and 6 months after intervention and then every 6 months, but such a rigid schedule may need adjustment to surgical observations and stability of the graft.
Tables and FiguresTop
|
Fontaine classification stage |
Symptoms |
Rutherford classification stage | |
|
I |
Asymptomatic |
|
0 |
|
IIa |
Claudication >200 meters |
Mild claudication |
1 |
|
IIb |
Claudication <200 meters |
Moderate claudication |
2 |
|
Severe claudication |
3 | ||
|
III |
Rest pain |
|
4 |
|
IV |
Ischemic necrosis or ulceration |
Minor tissue damagea |
5 |
|
Major tissue damageb |
6 | ||
|
a Nonhealing ulceration, focal gangrene with diffuse pedal ischemia. b Extending above the transmetatarsal level, functional foot no longer salvageable. | |||
|
Adapted from Helv Chir Acta. 1954;21(5-6):499-533 and J Vasc Surg. 1997;26(3):517-38. | |||
|
Condition |
Location of pain or discomfort |
Typical manifestations |
Onset of pain in relation to exercise |
Relation between rest and pain |
Relation between body position and pain |
Other features |
|
|
Claudication (hip, thigh, buttock) |
Hip, thigh, buttock |
Painful discomfort, weakness |
Always after the same defined type/amount of exercise |
Resolves rapidly |
None |
Pain can be induced in repeated manner |
|
|
Osteoarthritis of the hip |
Hip, thigh, buttock |
Painful discomfort |
After exercise of varied intensity |
Does not resolve quickly (pain may also be present at rest) |
Patient feels better when seated with lower extremities unweighted |
Variable pain, may depend on activity level or weather changes |
|
|
Spinal cord compression |
Hip, thigh, buttock (limited to dermatomes) |
Weakness dominates over pain |
After walking or standing for some time |
Resolves on stopping exercise only when accompanied by change in body position |
Patient feels relief after flexion of lumbar spine (when seated or leaning forward) |
In many patients history of back pain; pain induced by increase in abdominal pressure |
|
|
Claudication (lower leg/calf) |
Muscles of lower leg (calf) |
Cramp |
Always after the same defined type/amount of exercise |
Resolves rapidly |
None |
Pain can be induced in repeated manner |
|
|
Venous claudicationa |
Muscles of lower leg |
Severe, expanding |
After walking |
Resolves slowly |
Resolution faster when limb is elevated |
History of DVT, features of venous congestion, edema |
|
|
Radiculopathy (eg, herniated nucleus pulposus) |
Referred along lower extremity, usually in posterior aspects |
Severe, tearing |
Soon or immediately after compression |
Not resolving quickly (pain often present at rest) |
Resolution may occur upon changing spine position |
History of back pain |
|
|
Symptomatic popliteal (Baker) cyst |
In popliteal fossa, referred to lower leg |
Edema, pain, tenderness |
During exercise |
Pain at rest |
None |
Claudication not intermittent |
|
|
Claudication (foot) |
Foot |
Severe deep pain, numbness |
Always after the same defined type/amount of exercise |
Resolves rapidly |
None |
Pain can be induced in repeated manner |
|
|
|
Osteoarthritis or arthritis |
Foot |
Severe pain |
After exercise of varied intensity |
Not resolving quickly (pain may also be present at rest) |
Pain may be reduced by joint unweighting |
Variable pain, may depend on activity level |
|
a Bursting, heavy discomfort in legs with walking as legs become congested with blood due to impaired venous return. |
|||||||
|
Adapted from Eur J Vasc Endovasc Surg. 2000;19 Suppl A:Si-xxviii, S1-250. |
|||||||
|
DVT, deep vein thrombosis. |
|||||||
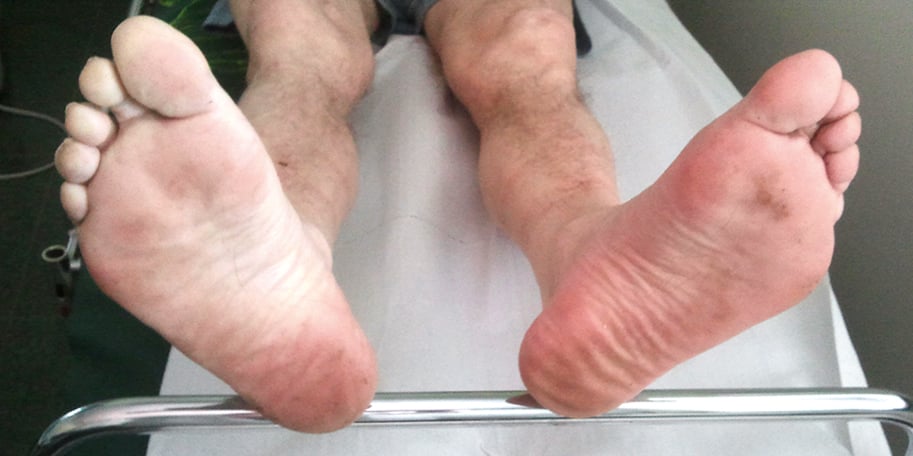
Figure 3.19-1. Foot pallor in an ischemic right leg upon slight elevation. Figure courtesy of Dr Leszek Masłowski.
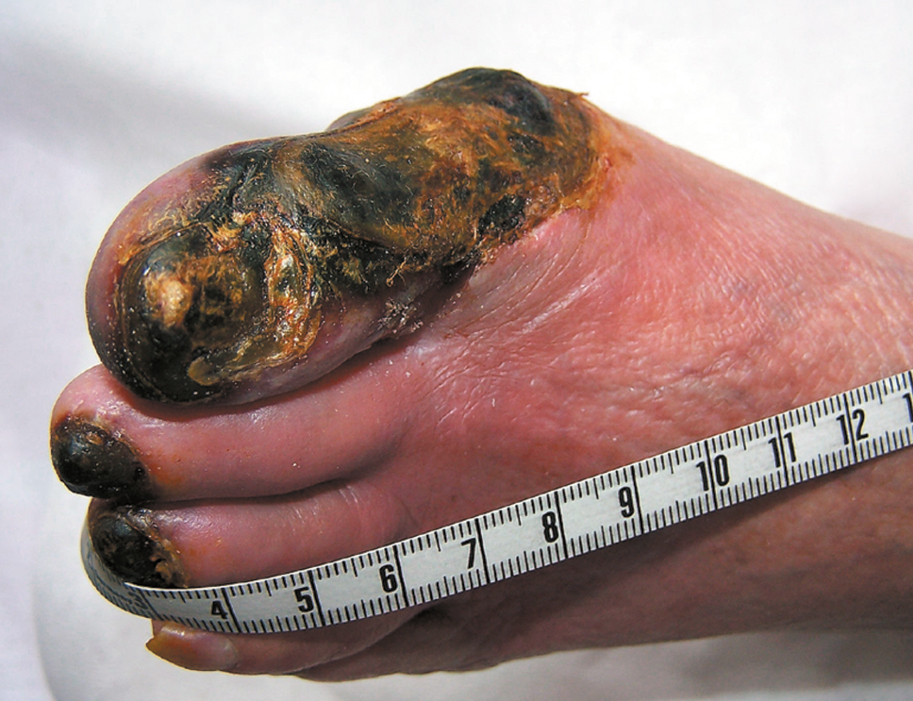
Figure 3.19-2. Dry gangrene of the toes due to chronic ischemia (critical ischemia). Figure courtesy of Dr Leszek Masłowski.
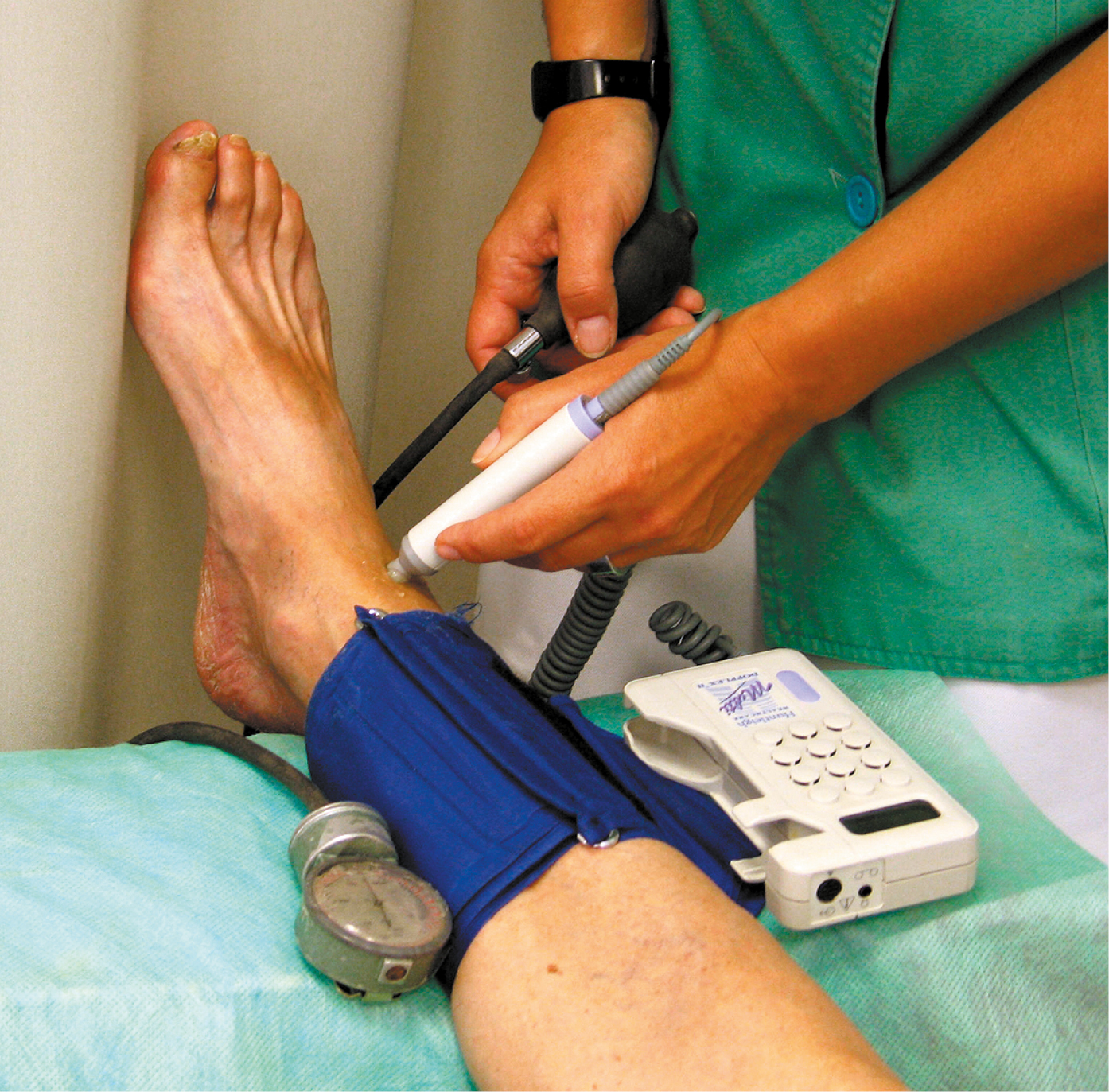
Figure 3.19-3. Ankle-brachial index.
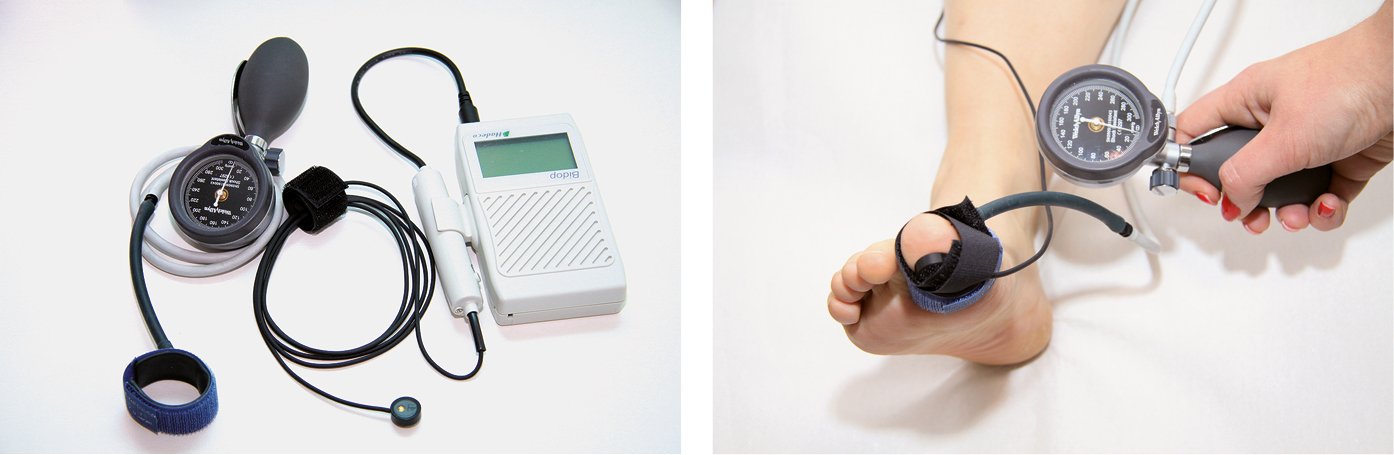
Figure 3.19-4. Toe-brachial index.