American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR). American Psychiatric Association Publishing; 2022.
Mühlbauer V, Möhler R, Dichter MN, Zuidema SU, Köpke S, Luijendijk HJ. Antipsychotics for agitation and psychosis in people with Alzheimer's disease and vascular dementia. Cochrane Database Syst Rev. 2021 Dec 17;12(12):CD013304. doi: 10.1002/14651858.CD013304.pub2. PMID: 34918337; PMCID: PMC8678509.
Brisson M, Brodeur C, Létourneau-Guillon L, et al. CCCDTD5: Clinical role of neuroimaging and liquid biomarkers in patients with cognitive impairment. Alzheimers Dement (N Y). 2021 Jan 22;6(1):e12098. doi: 10.1002/trc2.12098. PMID: 33532543; PMCID: PMC7821956.
Yunusa I, Alsumali A, Garba AE, Regestein QR, Eguale T. Assessment of Reported Comparative Effectiveness and Safety of Atypical Antipsychotics in the Treatment of Behavioral and Psychological Symptoms of Dementia: A Network Meta-analysis. JAMA Netw Open. 2019 Mar 1;2(3):e190828. doi: 10.1001/jamanetworkopen.2019.0828. PMID: 30901041; PMCID: PMC6583313.
Canadian Coalition for Seniors’ Mental Health. National Guidelines for The Assessment and Treatment of Mental Health Issues in Long Term Care Homes. Tool on Pharmacological Treatment of Behavioural Symptoms of Dementia in Long Term Care Facilities for Older Adults. www.ccsmh.ca/wp-content/uploads/2016/03/MHI-in-LTC-Final.pdf.
Dudas R, Malouf R, McCleery J, Dening T. Antidepressants for treating depression in dementia. Cochrane Database Syst Rev. 2018 Aug 31;8:CD003944. doi: 10.1002/14651858.CD003944.pub2. Review. PMID: 30168578.
Van Leeuwen E, Petrovic M, van Driel ML, et al. Withdrawal versus continuation of long-term antipsychotic drug use for behavioural and psychological symptoms in older people with dementia. Cochrane Database Syst Rev. 2018 Apr 1;3(3):CD007726. doi: 10.1002/14651858.CD007726.pub3. PMID: 29605970; PMCID: PMC8407230.
British Columbia Ministry of Health. Best Practice Guideline for Accommodating and Managing Behavioural and Psychological Symptoms of Dementia in Residential Care: A Person-Centered Interdisciplinary Approach. www.health.gov.bc.ca/library/publications/year/2012/bpsd-guideline.pdf. Published October 25, 2012. Accessed December 26, 2015.
Nelson JC, Devanand DP. A systematic review and meta-analysis of placebo-controlled antidepressant studies in people with depression and dementia. J Am Geriatr Soc. 2011 Apr;59(4):577-85. doi: 10.1111/j.1532-5415.2011.03355.x. Review. PMID: 21453380.
Ballard CG, Waite J, Birks J. Atypical antipsychotics for aggression and psychosis in Alzheimer’s disease. Cochrane Database Syst Rev. 2006 Jan 25;(1):CD003476. DOI: 10.1002/14651858.CD003476.pub2.
DefinitionTop
Dementia is a clinical syndrome characterized by progressive cognitive decline and impairment in ≥1 cognitive domain sufficient to cause decline in daily function. In 2013 the term “dementia” was subsumed under the newly named entity referred to in the fifth edition of the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders (DSM-5) as major neurocognitive disorders, which encompassed a group of acquired disorders. Mild neurocognitive disorder became in turn a DSM-5–recognized term for a less severe level of cognitive impairment (also referred to as mild cognitive impairment [MCI]), considered to be a potential prodrome of or risk factor for the development of Alzheimer disease or other neurodegenerative forms of dementia.
In the subsequent 2022 Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR), the terminology of neurocognitive disorders remained unchanged; however, its introduction does not mean that “dementia” is no longer used. Due to the term’s prevalence in both society and medical literature, we will continue to use “dementia” in this chapter.
Individuals with MCI or dementia can present with prominent and disabling behavioral disturbances, termed neuropsychiatric symptoms (NPSs) (previously behavioral and psychological symptoms of dementia [BPSD]). These include agitation, depression, apathy, delusions, hallucinations, and sleep impairment. In some cases they present as clusters, constituting syndromes such as dementia-associated psychotic or mood disturbances. These symptoms can have serious adverse consequences for patients and caregivers, such as greater impairment in activities of daily living, more rapid cognitive decline, worse quality of life, earlier institutionalization, and greater caregiver depression. Therefore, the NPSs of dementia are serious conditions that are increasingly becoming a focus of attention.
In this chapter we review the assessment and management of NPSs. Assessment, differential diagnosis, and management of cognitive symptoms in dementia: see Dementia.
EpidemiologyTop
NPSs can occur in >90% of patients with dementia, as an isolated symptom or in symptom clusters, and are often the presenting problems in seeking care. They are associated with more rapid disease progression, worse outcomes, and significant distress to the patient and caregivers.
The NPSs in patients with dementia may result from a number of precipitants or causal factors, including neurobiologically related disease factors; patient’s unmet needs (eg, too hot, too cold, hunger, incontinence, need to go to the bathroom); caregiver factors; environmental triggers; and interactions of the patient, caregiver, and environmental factors. It is likely that these potential causal factors are all relevant to differing degrees in an individual patient.
The cause of NPSs, while multifactorial, is likely due to regional brain degeneration. In late stages NPSs may be common to all dementias, irrespective of the etiology. Changes in the brain structure influence brain biology at multiple levels, including neural and neurotransmitter levels. Behaviors may result from these changes affecting the brain function or from the challenges with communication and environment. The traditional view of these behaviors as “challenging” to manage is gradually being replaced with the more person-centered terminology of these being responsive behaviors, in recognition that the behavior represents the brain’s attempts to communicate distress or unmet needs and should cue caregivers to determine potential triggers.
Clinical FeaturesTop
NPSs constitute a heterogeneous range of reactions, psychiatric symptoms, and behaviors that may impact the safety and care of patients with MCI or dementia. The most common NPSs in dementia (Table 16.9-8) and some of their key symptom presentations are further described below.
1. Agitation is one of the most pervasive symptoms and can occur as a primary symptom or secondary to other NPSs, including anxiety, depression, or psychosis. It may be associated with verbal (eg, repetitive vocalizations, shouting) or motor (eg, pacing, physical aggression, resistance to care) manifestations and can lead to injury, with significant implications for safety of the patient and others. Sundowning refers to a specific pattern of agitation, with worsening of agitation in the late afternoon or early evening. Onset of sundowning has been associated with decreased light exposure, timing of medications, and dysfunctional sleep-wake cycles.
2. Apathy may manifest as deficits in thinking, diminished ability to initiate action, and cognitive and emotional blunting. It tends to occur early and increase over the course of the illness. It is important to distinguish from depression, which, unlike apathy, causes great suffering and distress to the patient. Often more distressing to the caregiver than the patient, apathy can be associated with diminished self-care and lead to increased social isolation.
3. Psychotic symptoms in patients with dementia, such as hallucinations and delusions, also frequently occur in delirium, which must be excluded. Visual hallucinations are highly suggestive of delirium but also occur in neurodegenerative causes of parkinsonism, such as Parkinson disease, dementia with Lewy bodies, progressive supranuclear palsy, multiple system atrophy, and corticobasal degeneration syndrome (types of dementia: see Dementia). Paranoid delusions are the commonest, with themes of theft, infidelity, and misidentification syndromes as the most prominent. Psychotic symptoms can vary in intensity and severity, and if present without causing harm or distress to the patient or others, they may not require active pharmacologic treatment.
4. Depression has a high prevalence rate (15%-18%) in patients with dementia. Patients with dementia who have a past personal or family history of depression appear to be at greater risk for a major depressive episode, as do those with a history of stroke. Irritability and mood lability become more prevalent with the progression of dementia. Particularly, the apolipoprotein E epsilon 4 carriers with Alzheimer disease may have an increased risk of developing NPSs of depression and anxiety. Also see Depressive Disorders.
5. Disinhibition occurs in approximately one-third of patients and is more common with frontal lobe involvement. Sexually disinhibited or inappropriate behavior is often a source of great distress to caregivers. These behaviors can be caused by neurologic disorders, be associated with medication adverse effects (eg, dopamine agonists for Parkinson disease, benzodiazepines), be part of an undiagnosed bipolar disorder with mania, or occur as a symptom of dementia. Dementia-related disinhibited behaviors are important to distinguish from mania or hypomania, as this will have significant treatment implications. In individuals with a past personal or family history of bipolar or depressive disorder, a circumscribed trial of a mood stabilizer, if helpful, may provide diagnostic information, but this should not be done routinely and should prompt referral to a geriatric psychiatrist for diagnostic clarification and management.
6. Sleep-wake cycle disturbances are common and often associated with greater caregiver burden. Sleep disturbance is a common NPS in patients with dementia, who experience more sleep-wake cycle arousals and awakenings, have diminished rapid eye movement sleep, and take more daytime naps, exacerbating the problem.
DiagnosisTop
The diagnosis of NPSs includes a thorough evaluation of the following items:
1. An accurate history from both the patient and caregiver is an essential component to determine the cause or precipitant of the NPSs. Skill, patience, and flexibility are required to obtain accurate data. The clinician should face the patient directly and pay particular attention to eye contact, use unambiguous language, and speak in a calm, nonthreatening tone. Ongoing assessment for frustration or agitation of the patient is critical, as is recognizing when to switch topics or allow a break. The most important differential diagnosis is to exclude delirium as a cause for the behavioral change. Important elements of the history include:
1) Chronology and onset of the emergence of NPSs.
2) Sudden decline from baseline in function of instrumental and basic activities of daily living, suggesting delirium or acute cerebrovascular event.
3) Presence of a comorbid illness, especially signs and symptoms of delirium, vascular disease (risk factor for depression and agitation), infection, pain, and constipation.
4) Presence of past psychiatric episodes (eg, mood, psychosis, anxiety), which may represent a recurrence or relapse of underlying psychiatric disorder.
5) Presence of comorbid substance use disorder or withdrawal.
6) Medication adherence, changes, and withdrawal.
2. Physical examination, including a neurologic examination, is necessary to exclude infection, pain, constipation, and cerebrovascular or cardiovascular disease as a precipitant for the NPSs. A neurologic examination is important to exclude focal neurologic signs suggestive of stroke or subdural hematoma.
3. In patients with NPSs, the regular mental status examination should include a cognitive assessment, with a particular focus on change from previous testing. The most important task is to distinguish NPSs from delirium as a cause for the symptoms, particularly in cases of an acute change in attention. The presence of sadness, weepiness, agitation, suspiciousness or paranoia, disinhibition, or apathy should be documented. It can be challenging to distinguish depression as an NPS from a primary depressive disorder or a depressive episode of bipolar disorder. In both instances, the severity as well as degree of distress and impairment of function guide treatment. Cognitive assessment may include the use of the Mini-Mental State Examination (MMSE), which detects largely cortical deficits; however, the copyright restrictions make it less user-friendly (see Dementia). This should be supplemented by tests such as the Clock Drawing Test and the Montreal Cognitive Assessment (MoCA), which evaluates the frontal-subcortical executive function as well. The MoCA is available in multiple languages at mocacognition.com. Although MoCA is free for clinical use, it has been mandated that clinicians undergo the MoCA training and certification program to ensure validity and reliability of the test. In advanced stages of the illness many patients are unable to complete formal cognitive tests. In such cases it is important to rely on observations of behavior, speech, and response to stimuli to determine the level of cognitive impairment, noting that a change from baseline is more significant than the absolute test scores.
4. When NPSs occur in existing cognitive impairment but cannot be explained by the typical course of the underlying dementia, a formal neuropsychologic assessment can be a useful tool to help quantify impairment and monitor for change over time; however, it is resource intensive, expensive, and not recommended on a routine basis. The most commonly used scale for detection of NPSs of dementia is the Neuropsychiatric Inventory (NPI), which assesses symptoms in 12 domains (details: Table 16.9-9). The NPI-Clinician (NPI-C) version scale measures 14 domains and includes aberrant vocalization.
In patients initially presenting with NPSs early in the course of dementia or where diagnostic clarification will impact management, laboratory investigations are helpful to exclude other contributing or reversible factors. A summary of basic and more specific laboratory examinations performed to diagnose dementia: Table 16.9-10; details: see Dementia.
The differential diagnoses to be considered in a patient with cognitive impairment exhibiting NPSs include delirium, neurologic diseases, infections, metabolic abnormalities, depression, psychotic disorders, and drug-related mood or psychotic syndromes.
TreatmentTop
The treatment of NPSs can have a significant impact on the quality of life of the patient, their families, and caregivers. Referral to a geriatric psychiatrist, where available, a psychiatrist, or a geriatrician should be considered in instances of refractory symptoms that cause marked distress.
A useful approach to NPSs, which takes into account the likely precipitants or causal factors: Figure 16.9-1.
1. Nonpharmacologic treatment: The first line of treatment should always be to identify and address the underlying cause or precipitant of the NPS whenever possible (eg, pain, infection, constipation). Symptomatic treatment consists of nonpharmacologic strategies (Table 16.9-11) and should be the primary intervention for patients with dementia and NPSs, with clinicians and caregivers responding appropriately, knowledgeably, and promptly to address the patient’s needs while searching for the most likely trigger. Since there are fewer risks associated with these treatments, it is recommended that they should always be considered first,Evidence 1Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due to heterogeneity, imprecision (small sample size), and indirectness (population of volunteers, inability to separate different components of interventions). Livingston G, Johnston K, Katona C, Paton J, Lyketsos CG; Old Age Task Force of the World Federation of Biological Psychiatry. Systematic review of psychological approaches to the management of neuropsychiatric symptoms of dementia. Am J Psychiatry. 2005 Nov;162(11):1996-2021. Review. PMID: 16263837. Gitlin LN, Winter L, Dennis MP, Hodgson N, Hauck WW. Targeting and managing behavioral symptoms in individuals with dementia: a randomized trial of a nonpharmacologic intervention. J Am Geriatr Soc. 2010 Aug;58(8):1465-74. doi: 10.1111/j.1532-5415.2010.02971.x. PMID: 20662955; PMCID: PMC2955191. Legere LE, McNeill S, Schindel Martin L, Acorn M, An D. Nonpharmacologic approaches for behavioural and psychological symptoms of dementia in older adults: A systematic review of reviews. J Clin Nurs. 2018 Apr;27(7-8):e1360-e1376. doi: 10.1111/jocn.14007. Review. PMID: 28793380. tailored to the individual patient, and their impact should be carefully monitored through the use of standardized behavioral assessment tools such as the Cohen-Mansfield Agitation Inventory. Even when specific etiologies are identified, nonpharmacologic interventions should be used in combination with medication (Figure 16.9-1). Family and caregivers are key collaborators and need to be involved in treatment planning. Offering education to families and caregivers (both formal and informal) is essential. By making dementia education more accessible, online toolkits and material can help manage issues at home before a crisis occurs. For example, iGeriCare is a free online dementia education program developed by experts in psychiatry, geriatrics, and online learning at McMaster University, and is complementary to the traditional groups from other organizations such as the Alzheimer Society of Canada.
2. Pharmacologic treatment: General prescribing principles: Prior to prescribing, in nonemergent situations it is imperative to inform patients or their substitute decision-makers of pertinent risks and warnings issued regarding medication (discussed below), to weigh the benefits versus the risks, and to document informed consent in the patient’s chart. Age-related changes in pharmacodynamics and pharmacokinetics of psychotropic medications (eg, longer time to reach steady state, longer half-life, and longer elimination time) should be taken into consideration. Lower doses, cautious dose adjustments, and regular reassessment of the need for continuing the treatment in the geriatric patients should be considered. Geriatric patients with dementia are more vulnerable to adverse effects, including sedation, anticholinergic adverse effects, cognitive decline, extrapyramidal symptoms, and drug-drug interactions. Agitation, which is often the presenting symptom of an underlying mood, anxiety, or psychotic disorder, is the most common NPS requiring specific pharmacologic treatment after other treatments have failed. Below is a summary of the options that can be used for NPSs.
1) Antidepressants: The evidence for benefits of antidepressants in patients with dementia is modest; however, clinical guidelines suggest a trial of an antidepressant when there is a high index of suspicion for depression causing distress (despite not meeting criteria for major depressive disorder), while monitoring for adverse effects. Adverse events associated with antidepressants include hyponatremia, bleeds, falls, fragility, and osteoporotic fractures, and their rates are fairly low. It should be noted that at higher doses, selective serotonin reuptake inhibitors (SSRIs) can cause affective flattening and worsen apathy. In patients partially responsive to SSRIs or with prominent apathy, cautious use of low-dose psychostimulants (eg, methylphenidate, atomoxetine, modafinil) may be suggested, particularly in individuals who are both profoundly apathetic and medically ill.Evidence 2Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). Low Quality of Evidence (low confidence that we know true effects of the intervention). Quality of Evidence lowered due to heterogeneity, imprecision, small number of studies within each drug class, risk of bias, publication bias, and inconsistency between studies. Ruthirakuhan MT, Herrmann N, Abraham EH, Chan S, Lanctôt KL. Pharmacologic interventions for apathy in Alzheimer’s disease dementia. Cochrane Database Syst Rev. 2018 May 4;5:CD012197. doi: 10.1002/14651858.CD012197.pub2. Review. PMID: 29727467. Because psychostimulants can induce elevation of blood pressure and heart rate, irritability, agitation, and psychosis, careful patient selection is critical, especially in individuals with severe cardiovascular disease or other underlying cardiac conditions.
Antidepressants have limited evidence for efficacy,Evidence 3Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). Low Quality of Evidence (low confidence that we know true effects of the intervention). Quality of Evidence lowered due to heterogeneity and imprecision. Dudas R, Malouf R, McCleery J, Dening T. Antidepressants for treating depression in dementia. Cochrane Database Syst Rev. 2018 Aug 31;8:CD003944. doi: 10.1002/14651858.CD003944.pub2. Review. PMID: 30168578. especially beyond 12 weeks, in the treatment of NPSs of dementia of any type other than major depression, but there are clinical scenarios where a trial of an antidepressant may be appropriate. Patients with dementia may develop NPSs of apathy, social withdrawal, and sleep disturbance that may suggest the presence of depression but that are NPSs entirely due to dementia. Given the degree of suffering in untreated depression, a therapeutic trial with an antidepressant may be a reasonable diagnostic strategy in such cases. A low threshold for initiating antidepressant treatment should be considered in patients with preexisting history of depression or significant cerebrovascular disease. SSRIs are the preferred antidepressants in treating depression in patients with dementia and are generally well tolerated. Selection of an antidepressant is usually based upon the adverse-effect profile (particularly anticholinergic), drug-drug interactions, medical comorbidities, and cost.
SSRIs may also be useful in the management of agitation and psychosis in patients with Alzheimer disease, particularly if poorly articulated depression is a likely precipitant of the NPS manifestation or cannot be excluded when no other cause is apparent.Evidence 4Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). Low Quality of Evidence (low confidence that we know true effects of the intervention). Quality of Evidence lowered due to heterogeneity and imprecision. Seitz DP, Adunuri N, Gill SS, Gruneir A, Herrmann N, Rochon P. Antidepressants for agitation and psychosis in dementia. Cochrane Database Syst Rev. 2011 Feb 16;(2):CD008191. doi: 10.1002/14651858.CD008191.pub2. Review. PMID: 21328305. Schneider LS, Frangakis C, Drye LT, et al; CitAD Research Group. Heterogeneity of Treatment Response to Citalopram for Patients With Alzheimer's Disease With Aggression or Agitation: The CitAD Randomized Clinical Trial. Am J Psychiatry. 2016 May 1;173(5):465-72. doi: 10.1176/appi.ajp.2015.15050648. PMID: 26771737. Although having limited efficacy, the antidepressant citalopram has been shown to be as effective as risperidone, a second-generation antipsychotic (SGA), in the treatment of agitation due to dementia. Patients who are more likely to benefit from citalopram have moderate agitation and lower levels of cognitive impairment. Citalopram should be avoided in patients at increased risk for arrhythmias and discontinued in the case of a persistent QTc >500 ms. Because of the association of citalopram with cardiac QTc prolongation, use of this medication to treat agitation may be limited to a subgroup of patients with dementia. Health Canada and the United States Food and Drug Administration (FDA) recommend for citalopram a maximum daily dose of 20 mg for patients aged ≥60 years. The use of SSRIs for an indication of NPSs other than depression necessitates ongoing assessment of benefits versus risks, and consideration for withdrawing the medications should be made periodically. Several other antidepressants were not found to be useful for agitation. However, sertraline at daily doses 25 to 100 mg can have a modest benefit on agitation.
Some evidence suggests that the NPSs in frontotemporal dementia may be caused by deficits in the serotoninergic system, in particular the repetitive behaviors. There is limited evidence of benefit for serotonergic antidepressant agents in these patients (Table 16.9-12). Given the absence of evidence for using any other pharmacologic agents in this illness, a trial of an SSRI in these patients is warranted if the NPSs are causing distress.
2) Cholinesterase inhibitors: Cholinesterase inhibitors have only modest benefits overall for the treatment of NPSs. In individuals with dementia due to Alzheimer disease or dementia with Lewy bodies, cholinesterase inhibitors may be used for the treatment of apathy, anxiety, and depression, as well as disinhibition and aberrant motor symptoms. Cholinesterase inhibitors are not recommended as treatment for agitation in patients with dementia because they may worsen the agitation,Evidence 5Weak recommendation (downsides likely outweigh benefits, but the balance is close or uncertain; an alternative course of action may be better for some patients). Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due to imprecision, indirectness, and short duration of the study (12 weeks). Howard RJ, Juszczak E, Ballard CG, et al; CALM-AD Trial Group. Donepezil for the treatment of agitation in Alzheimer's disease. N Engl J Med. 2007 Oct 4;357(14):1382-92. PMID: 17914039. particularly in patients with frontotemporal dementia.
3) Anticonvulsant mood stabilizers: Treatment with anticonvulsant mood stabilizers (eg, carbamazepine, valproic acid, gabapentin) in patients with dementia and NPSs can be considered, but use of these agents should be limited. Carbamazepine can have significant adverse events (particularly in geriatric patients) such as sedation, hyponatremia, and cardiac toxicity, and is a strong enzymatic inducer, with a high likelihood of drug-drug interactions, making the risk-benefit ratio too high to be considered on a routine basis. Valproic acid is less recommended due to very limited efficacy on these symptoms. In general, anticonvulsants are not routinely used in the treatment of NPSs, unless the patient has a comorbid diagnosis of bipolar disorder.
4) Antipsychotics: Psychosis and aggression in dementia requiring pharmacotherapy with antipsychotics may respond best to risperidone, olanzapine, and aripiprazole. The use of haloperidol should be limited to addressing agitation or psychosis in delirium only, using low doses for a brief period of time until symptoms clear. When the target problem behaviors are wandering, social withdrawal, vocalizing, pacing, touching, or incontinence, antipsychotics are not usually effective and are thus not recommended.
If the behavioral disturbances such as agitation or aggression are not part of a psychotic disorder or bipolar mania but rather nonspecific symptoms of dementia, antipsychotics have modest benefits at best. While antipsychotics should be used judiciously, the apparent effectiveness of the medications seen in daily practice may instead be explained by a favorable natural course of the symptoms. The serious adverse effects of these agents, such as increased risk of cerebrovascular events and mortality, necessitate the use of antipsychotic medications as a last resort to treat NPSs of dementia and only if nondrug therapies have failed to be effective and the patients’ actions threaten the patients or others.Evidence 6Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due to imprecision and short duration of studies. Mühlbauer V, Möhler R, Dichter MN, Zuidema SU, Köpke S, Luijendijk HJ. Antipsychotics for agitation and psychosis in people with Alzheimer's disease and vascular dementia. Cochrane Database Syst Rev. 2021 Dec 17;12(12):CD013304. doi: 10.1002/14651858.CD013304.pub2. PMID: 34918337; PMCID: PMC8678509. Sultzer DL, Davis SM, Tariot PN, et al; CATIE-AD Study Group. Clinical symptom responses to atypical antipsychotic medications in Alzheimer's disease: phase 1 outcomes from the CATIE-AD effectiveness trial. Am J Psychiatry. 2008 Jul;165(7):844-54. doi: 10.1176/appi.ajp.2008.07111779. PMID: 18519523; PMCID: PMC2714365. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005 Oct 19;294(15):1934-43. PMID: 16234500. Gill SS, Rochon PA, Herrmann N, et al. Atypical antipsychotic drugs and risk of ischaemic stroke: population based retrospective cohort study. BMJ. 2005 Feb 26;330(7489):445. PMID: 15668211; PMCID: PMC549652. Notably, from 2005 onwards, regulatory agencies in North America, Europe, and Australia have issued black box warnings on the increased risk of cerebrovascular events and mortality in patients with dementia receiving either first-generation antipsychotics (FGAs), SGAs, or third-generation antipsychotics (TGAs). However, Health Canada indicates the use of an SGA, risperidone, for “short-term symptomatic management of aggression or psychotic symptoms in patients with severe dementia of the Alzheimer type unresponsive to nonpharmacologic approaches and when there is a risk of harm to self or others.” Similarly to aripiprazole, brexpiprazole is a TGA as well, with dopamine D2 and serotonin 5-HT1A partial agonism and antagonist activity at serotonergic 5-HT2A receptors. In 2024 Health Canada approved the use of brexpiprazole for symptomatic management of agitation associated with dementia due to Alzheimer disease. Brexpiprazole is not recommended as an as-needed (prn) treatment for agitation; this is due to the fact that up to 6 to 8 weeks may be needed after brexpiprazole initiation to demonstrate clinical efficacy.Evidence 7Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due to the risk of bias, imprecision, and short duration of studies (12 weeks). Lee D, Slomkowski M, Hefting N, et al. Brexpiprazole for the treatment of agitation in Alzheimer dementia: A randomized clinical trial. JAMA Neurol. 2023 Dec 1;80(12):1307-1316. doi: 10.1001/jamaneurol.2023.3810. PMID: 37930669; PMCID: PMC10628834. Health Canada. Product Monograph Including Patient Medication Information: PrREXULTI® Brexpiprazole tablets. Otsuka Pharmaceutical Co., Ltd. Reviewed January 23, 2024. Accessed August 2024. https://health-products.canada.ca/dpd-bdpp/info?lang=eng&code=94955 The use of antipsychotics requires individualized risk-benefit evaluation in collaboration with patients’ families. Common adverse events of antipsychotics include sedation; gait changes; increased risk of falls and fracture; extrapyramidal symptoms including parkinsonism, dystonias, and akathisia (particularly with antipsychotics with high D2 blockade, such as haloperidol, risperidone, and aripiprazole); and metabolic adverse effects of weight gain and dyslipidemia (higher risk in female patients, with olanzapine and quetiapine). Longer-term use of FGAs (and some SGAs and TGAs) carries the risk of tardive dyskinesia, particularly in older female patients with dementia and comorbid depressive or bipolar disorders. Because there is no single most effective and safe treatment option, physicians prescribing antipsychotics to patients with dementia should trade off between the effectiveness and safety of these medications in the treatment of NPSs.Evidence 8Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due to imprecision and short duration of studies (indirectness). Yunusa I, Alsumali A, Garba AE, Regestein QR, Eguale T. Assessment of Reported Comparative Effectiveness and Safety of Atypical Antipsychotics in the Treatment of Behavioral and Psychological Symptoms of Dementia: A Network Meta-analysis. JAMA Netw Open. 2019 Mar 1;2(3):e190828. doi: 10.1001/jamanetworkopen.2019.0828. PMID: 30901041; PMCID: PMC6583313.
Most behavioral symptoms of dementia are intermittent and often do not persist for >3 months. Therefore, when antipsychotic treatment is instituted, regular attempts to withdraw these medications are recommended.Evidence 9Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). Low Quality of Evidence (low confidence that we know true effects of the intervention). Quality of Evidence lowered due to heterogeneity and imprecision (few studies and small subgroups). Van Leeuwen E, Petrovic M, van Driel ML, et al. Withdrawal versus continuation of long-term antipsychotic drug use for behavioural and psychological symptoms in older people with dementia. Cochrane Database Syst Rev. 2018 Apr 1;3(3):CD007726. doi: 10.1002/14651858.CD007726.pub3. PMID: 29605970; PMCID: PMC8407230. Generally, stopping antipsychotics for patients with dementia does not cause problems, even in those with long-term use, and it is suggested that discontinuation programs should be incorporated into routine practice. Therefore, antipsychotics can be safely discontinued in specific cases without short-term worsening of behavioral symptoms. Predictors of successful discontinuation include lower daily doses of antipsychotics and lower baseline severity of behavioral symptoms. A small subgroup of patients with more severe NPSs at baseline may be unable to tolerate discontinuation of antipsychotics and will require long-term treatment. It remains uncertain whether discontinuation of antipsychotics leads to a decrease in mortality.
5) Benzodiazepines: Despite widespread use, there is lack of evidence for prescribing benzodiazepines safely in older adults. The risks clearly outweigh the benefits, where large-scale studies consistently show that the risk of worsening cognition, falls, and hip fractures leading to hospitalization and death can more than double in older adults taking benzodiazepines. According to Choosing Wisely Canada, the use of benzodiazepines should be reserved for alcohol withdrawal symptoms in delirium tremens or severe generalized anxiety disorder unresponsive to other therapies; other acceptable indications would be neuroleptic malignant syndrome or catatonia. Outside of these specific indications, benzodiazepines should not be used as a first-line agent in treating agitation or other behavioral disturbances in patients with NPSs of dementia. Initiating (risk of dependence) or discontinuing (risk of withdrawal) sedative-hypnotics in hospital can have substantial impact on their long-term use. Short-term use, on-demand use (as needed), or use as a chemical restraint of shorter-acting benzodiazepines, such as lorazepam (often in conjunction with haloperidol), can be considered to address severe agitation or aggression. In practice, many older adults who present with NPSs have been on benzodiazepines for decades and are likely to continue to need this class of drugs; however, the goal is to taper and use the lowest doses of short-acting or intermediate-acting agents (lorazepam, clonazepam) that are effective. Ultra-short-acting agents, such as alprazolam, are not recommended due to their amnestic and significant dependence effects.
6) Disease-modifying agents (anti-amyloid monoclonal antibodies): Monoclonal antibodies, lecanemab and aducanumab, have shown promising results through their disease-modifying mechanism to slow the progress of Alzheimer disease when initiated in mild cognitive impairment with the confirmatory presence of amyloid beta plaques and mild dementia stage. Currently, no disease-modifying treatments for the treatment of Alzheimer disease have been approved by Health Canada, although lecanemab is under review. Donanemab, another monoclonal antibody, has shown promise in patients with prodromal to moderate dementia due to Alzheimer disease in a phase 3 clinical trial. The available evidence of effectiveness of these disease-modifying agents for improving the course of Alzheimer disease is limited. A robust postmarketing adverse effect surveillance program for these treatments is essential in order to weigh the modest benefits (ie, moderately lower decline on measures of cognition and function at 18 months) against safety concerns (eg, infusion-related reactions, cerebral edema, intracerebral hemorrhage). Therefore, whether the use of monoclonal antibodies is the game changer remains to be seen. Nevertheless, little is known about whether these monoclonal antibodies have any effect on the NPS status. The heterogeneity of the disease may require personalized approaches and perhaps combination therapies to maximize therapeutic efficacy.Evidence 10Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due to limitations of performed studies and limited generalizability beyond trial participant pools; longer-term follow-up studies are critical to assess the durability of treatment effects and its potential to provide cumulative benefits. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023 Jan 5;388(1):9-21. doi: 10.1056/NEJMoa2212948. Epub 2022 Nov 29. PMID: 36449413. Watt JA, Isaranuwatchai W, Grossman L, Straus SE. Disease-modifying drugs for Alzheimer disease: implications for people in Canada. CMAJ. 2023 Oct 30;195(42):E1446-E1448. doi: 10.1503/cmaj.230595. PMID: 37903526; PMCID: PMC10615338. Brockmann R, Nixon J, Love BL, Yunusa I. Impacts of FDA approval and Medicare restriction on antiamyloid therapies for Alzheimer's disease: patient outcomes, healthcare costs, and drug development. Lancet Reg Health Am. 2023 Mar 1;20:100467. doi: 10.1016/j.lana.2023.100467. PMID: 36908502; PMCID: PMC9996432.
A more comprehensive review of the common pharmacologic agents used in the management of NPSs in dementia: Table 16.9-12.
Some common pharmacologic options to use versus “better-not-to-use” pharmacologic options for the main NPS clusters in dementia: Table 16.9-13.
Tables and FiguresTop
|
– Apathy – Depression – Elevated mood – Irritability – Mood lability – Anxiety – Disruptive vocalization – Aggression – Agitation – Sundowning – Illusions and hallucinations – Delusions – Sleep disturbances – Wandering – Pacing – Hoarding – Inappropriate sexualized behavior |
|
– Delusions – Hallucinations – Agitation – Dysphoria – Anxiety – Apathy – Irritability – Euphoria – Disinhibition – Aberrant motor behavior – Night-time behavior disturbances – Appetite and eating abnormalities |
|
Basic screening investigations |
– Complete blood count, thyroid-stimulating hormone, serum electrolytes, eGFR, serum calcium, and serum glucose – Screening for depression (eg, Geriatric Depression Scale) |
|
Specific examinations (if justified) |
– Heavy metal screens – VDRL test – Routine genetic markers (eg, apolipoprotein E) are not recommended |
|
ECG |
– Screening for vascular risk factors |
|
Lumbar puncture |
– For specific concerns (eg, infection, demyelinating diseases, CSF 14-3-3 protein in Creutzfeldt-Jakob disease) – CSF biomarkers of Alzheimer disease pathology (CSF Abeta1-42 and tau) have no clinical utility in Canada but are part of research protocols |
|
EEG |
– Creutzfeldt-Jakob disease or dementia with seizures can be identified |
|
Structural brain imaging (CT or MRI) |
– CCCDTD5 recommends that structural neuroimaging is indicated in most but not required in all patients with cognitive impairment (recommendations for neuroimaging and specific clinical features: see Dementia) – Although more costly, MRI is preferable to CT – CT or MRI should be undertaken in the assessment of cognitive impairment if the presence of unsuspected cerebrovascular disease would change the clinical management |
|
Functional brain imaging (FDG-PET, tau-PET SPECT) |
– Where available, FDG-PET or PET amyloid imaging can be used for clinical purposes in patients with atypical dementias – 18F-florbetaben FDG-PET can be recommended for differential diagnosis purposes if the underlying pathologic process remains unclear after a baseline evaluation, preventing adequate clinical management. If FDG-PET is unavailable, a SPECT study can be recommended – Tau-PET with 18F-flortaucipir is indicated for imaging of the brain to estimate the density and distribution of aggregated tau neurofibrillary tangles in adult patients with cognitive impairment who are being evaluated for Alzheimer disease. Flortaucipir was the first tau-PET tracer to be introduced and has been widely adopted and validated in several research and clinical settings – SPECT with 123I-ioflupane (DaTscanTM) can help differentiate Alzheimer disease from Lewy body disease |
|
CCCDTD5, 5th Canadian Consensus Conference on the Diagnosis and Treatment of Dementia; CSF, cerebrospinal fluid; CT, computed tomography; ECG, electrocardiography; EEG, electroencephalography; eGFR, estimated glomerular filtration rate; FDG-PET, 18F-fluorodeoxyglucose positron emission tomography; MRI, magnetic resonance imaging; PET, positron emission tomography; SPECT, single-photon emission computed tomography; tau, a neuronal protein that aggregates in Alzheimer disease; VDRL, venereal disease research laboratory. |
|
|
Sensory enhancement/relaxation |
Social contact: real or simulated |
Behavior therapy |
|
– Massage and touch – Individualized music – White noise – Controlled multisensory stimulation (Snoezelen) – Art therapy – Aromatherapy |
– Individualized social contact – Pet therapy – 1:1 social interaction – Simulated interactions/family videos |
– Differential reinforcement – Stimulus control |
|
Structured activities |
Environmental modifications |
Training and development |
|
– Recreational activities – Outdoor walks – Physical activities |
– Wandering areas – Natural/enhanced environments – Reduced stimulation – Light therapy |
– Staff education – Staff support – Training programs for family caregivers |
|
Medication category |
Daily dose |
Adverse-effect monitoring |
Comments |
|
Second-generation and third-generation antipsychotics (SGAs and TGAs) |
|||
|
Risperidone (LAI, ODT, liquid) |
Initial: 0.25 mg once daily to bid Titration: 0.25-0.5 mg every 3-7 days Max: 2 mg |
– Sedation – Postural hypotension – Falls – Anticholinergic adverse effects (dry mouth, constipation, confusion) – EPS, particularly parkinsonian signs and symptoms (rigidity, bradykinesia, shuffling gait, masked facies, tremor) – Olanzapine and quetiapine more sedating than risperidone, aripiprazole, or brexpiprazole |
– Best supported SGA for NPSs – Most likely SGA/TGA to cause EPS |
|
Olanzapine (IM, ODT) |
Initial: 2.5-5 mg at bedtime Titration: 2.5-5 mg every 3-7 days Max: 10 mg |
– Most likely SGA/TGA to cause metabolic adverse effects – Availability in rapidly dissolving preparation advantageous in noncompliance |
|
|
Quetiapine |
Initial: 12.5 mg bid Titration: 12.5-25 mg every 3-7 days Max: 150 mg |
– Used for Parkinson disease dementia and dementia with Lewy bodies at lower doses due to high sensitivity to EPS – In those with psychosis and Parkinson disease dementia, if possible, first reduce dopaminergic agents |
|
|
Aripiprazole |
Initial: 2-5 mg daily Titration: 2-5 mg every 3-7 days Max: 10 mg |
Most likely SGA/TGA to cause akathisia (restlessness) |
|
|
Brexpiprazole
|
Initial: 0.5 mg daily Titration: 0.5 mg every 7 days Max: 3 mg |
Most recent antipsychotic approved to treat agitation associated with dementia due to Alzheimer disease |
|
|
Pimavanserin |
Initial: 10 mg Max: 34 mg
|
Nausea, constipation, peripheral edema, gait disturbances |
– Selective inverse agonist-antagonist of the 5-HT2A receptor – Approved in the US for Parkinson disease psychosis; however, subgroup analysis of the HARMONY trial showed a significantly reduced risk of psychosis relapse in patients with Parkinson disease dementia |
|
First-generation antipsychotics (FGAs) |
|||
|
Haloperidol (IM, IV, LAI, liquid) |
Initial: 0.25 mg bid Titration: 0.5 mg bid every 3-7 days Max: 1.5 mg bid |
Haloperidol more likely to cause EPS than SGAs |
– Gold standard for delirium – Can be given IM in ED when other formulations unavailable or IV in ICU; monitor ECG for QTc prolongation, especially when dosage ≥3 mg/d |
|
SSRI antidepressants |
|||
|
Citalopram |
Initial: 5-10 mg daily Titration: 10 mg every 7 days Max: 20 mg |
– Headache – Nausea (give with food to ↓GI upset) – Diarrhea – Sweating – Insomnia – Hyponatremia – Risk of GI bleed – QTc prolongation at higher doses of citalopram – Risk of falls, fractures, and osteoporosis |
– Citalopram is the best supported SSRI for NPS; monitor ECG for QTc prolongation, especially when dosage ≥20 mg/d – In FTD patients, SSRIs are first-line agents, particularly for repetitive behaviors |
|
Escitalopram |
Initial: 5 mg daily Titration: 5 mg every 7 days Max: 10 mg |
||
|
Sertraline |
Initial: 25 mg daily Titration: 25 mg every 7 days Max: 100 mg |
||
|
Anticonvulsants |
|||
|
Carbamazepine |
Initial: 50 mg daily Titration: 50 mg every 7-14 days, bid to tid Max: 500 mg |
– Sedation – Ataxia/falls – Neutropenia – Hyponatremia – ↑ Liver function tests – Skin rash |
– Risk of drug-drug interactions – Monitor drug level |
|
Agents for sleep |
|||
|
Lorazepam (IM, IV, liquid) |
Initial: 0.25-0.5 mg at bedtime Titration: 0.25-0.5 mg every 3-7 days Max: 2 mg |
BZPs: – Sedation – Confusion/cognitive impairment – Ataxia/falls – Disinhibition Trazodone: – Postural hypotension – Dry mouth – Constipation |
– BZPs for short-term use only – BZPs carry risk of tolerance/dependence, falls, worsening cognition, and withdrawal upon discontinuation; their use should be limited to situations that may require rapid onset of action while under close observation – Trazodone can be used in FTD, in 25 mg bid to tid starting dose to 100-300 mg daily |
|
Zopiclone |
Initial: 3.75 mg at bedtime Titration: 3.75 mg every 3-7 days Max: 15 mg |
||
|
Trazodone |
Initial: 25 mg at bedtime Titration: 25 mg every 3-7 days Max: 100 mg |
||
|
Cholinesterase inhibitors |
|||
|
Donepezil |
Initial: 2.5-5 mg daily Titration: 2.5-5 mg every 4-6 weeks Max: 10 mg (23 mg slow release) |
– GI upset (nausea/vomiting/diarrhea) – Loss of appetite – ↓ GI adverse effects with patch – Insomnia, hypervivid dreams – Bradycardia – Urinary incontinence – Muscle cramps |
– ChEIs are first-line agents for psychosis in Parkinson disease dementia and dementia with Lewy bodies – ChEIs can be beneficial in apathy – Take with food to minimize GI upset – Rotate patch site – In FTD, ChEIs are contraindicated |
|
Rivastigmine |
Initial: 1.5 mg bid Titration: 1.5 mg every 4 weeks Max: 12 mg |
||
|
Initial: 4.6 mg patch Titration: 9.5 mg every 4 weeks Max: 13.3 mg |
|||
|
Galantamine |
Initial: 8 mg daily Titration: 8 mg every 4 weeks Max: 24 mg |
Extended-release formulation |
|
|
bid, 2 times a day; BZP, benzodiazepine; ChEI, cholinesterase inhibitor; ECG, electrocardiogram; ED, emergency department; EPS, extrapyramidal symptoms; FGA, first-generation antipsychotic; FTD, frontotemporal dementia; GI, gastrointestinal; ICU, intensive care unit; IM, available as intramuscular formulation; IV, available as intravenous formulation; LAI, available as long-acting injectable formulation; NPS, neuropsychiatric symptom; ODT, available as orally dissolving/disintegrating tablet; SGA, second-generation antipsychotic; SSRI, selective serotonin reuptake inhibitor; tid, 3 times a day; TGA, third-generation antipsychotic; US, United States. |
|||
|
NPSs in dementia |
Pharmacologic options |
“Better-not-to-use” pharmacologic options |
|
Agitation/aggression |
– Serotonergic antidepressants (eg, citalopram, sertraline, trazodone) – Shorter-acting BZPs (eg, lorazepam, oxazepam) – Anticonvulsants (eg, carbamazepine, valproic acid) if comorbid bipolar disorder – SGAs/TGAs (eg, risperidone, olanzapine, quetiapine, aripiprazole, brexpiprazole) – FGAs (eg, haloperidol) |
– Do not use TGAs/SGAs/FGAs as first choice – Avoid highly anticholinergic FGAs/SGAs (eg, chlorpromazine, perphenazine, clozapine) – Avoid highly dopamine-blocking FGAs and SGAs in parkinsonism-related dementias (eg, haloperidol, risperidone) – Limit use of anticonvulsants (eg, carbamazepine, valproic acid) if no comorbid bipolar disorder – ChEIs may worsen agitation; do not use in FTD – Avoid BZDs/sedative-hypnotics as first choice |
|
Apathy |
– ChEIs (eg, donepezil, rivastigmine, galantamine) – Antidepressants – Psychostimulants (eg, methylphenidate, modafinil) |
– FGAs/SGAs may worsen apathy – Antidepressants at high doses may worsen apathy |
|
Psychosis |
– ChEIs (eg, donepezil, rivastigmine, galantamine) – SGAs/TGAs – FGAs |
– Limit use of FGAs and most of TGAs and SGAs (except quetiapine) in dementia with Lewy bodies and Parkinson disease dementia due to worsening of EPSs in dose-dependent fashion – Limit use of FGAs/SGAs/TGAs in prolonged QTc syndrome (except aripiprazole) |
|
Depression |
– Antidepressants (eg, citalopram, escitalopram, sertraline, venlafaxine, bupropion, mirtazapine) |
– Avoid highly anticholinergic antidepressants (eg, clomipramine, amitriptyline, doxepin) |
|
Disinhibition |
– Antidepressants (eg, citalopram, trazodone) – Antiandrogens (eg, medroxyprogesterone acetate) – GnRH analogues (eg, leuprolide) – SGAs/FGAs (eg, quetiapine, haloperidol) |
– BZPs may worsen disinhibition – Dopamine agonists may worsen disinhibition |
|
Sleep disturbances |
– Antidepressants (eg, mirtazapine, trazodone) – Shorter-acting BZPs/sedative-hypnotics, if necessary, for a brief period of time (eg, lorazepam, oxazepam, temazepam, zopiclone) |
– Avoid BZDs/sedative-hypnotics as first choice – Do not use long-acting BZPs (eg, diazepam, chlordiazepoxide, flurazepam) due to drug accumulation, active metabolites |
|
BZP, benzodiazepine; ChEI, cholinesterase inhibitor; EPS, extrapyramidal symptom; FGA, first-generation antipsychotic; FTD, frontotemporal dementia; GnRH, gonadotropin-releasing hormone; NPS, neuropsychiatric symptom; SGA, second-generation antipsychotic; TGA, third-generation antipsychotic. |
||
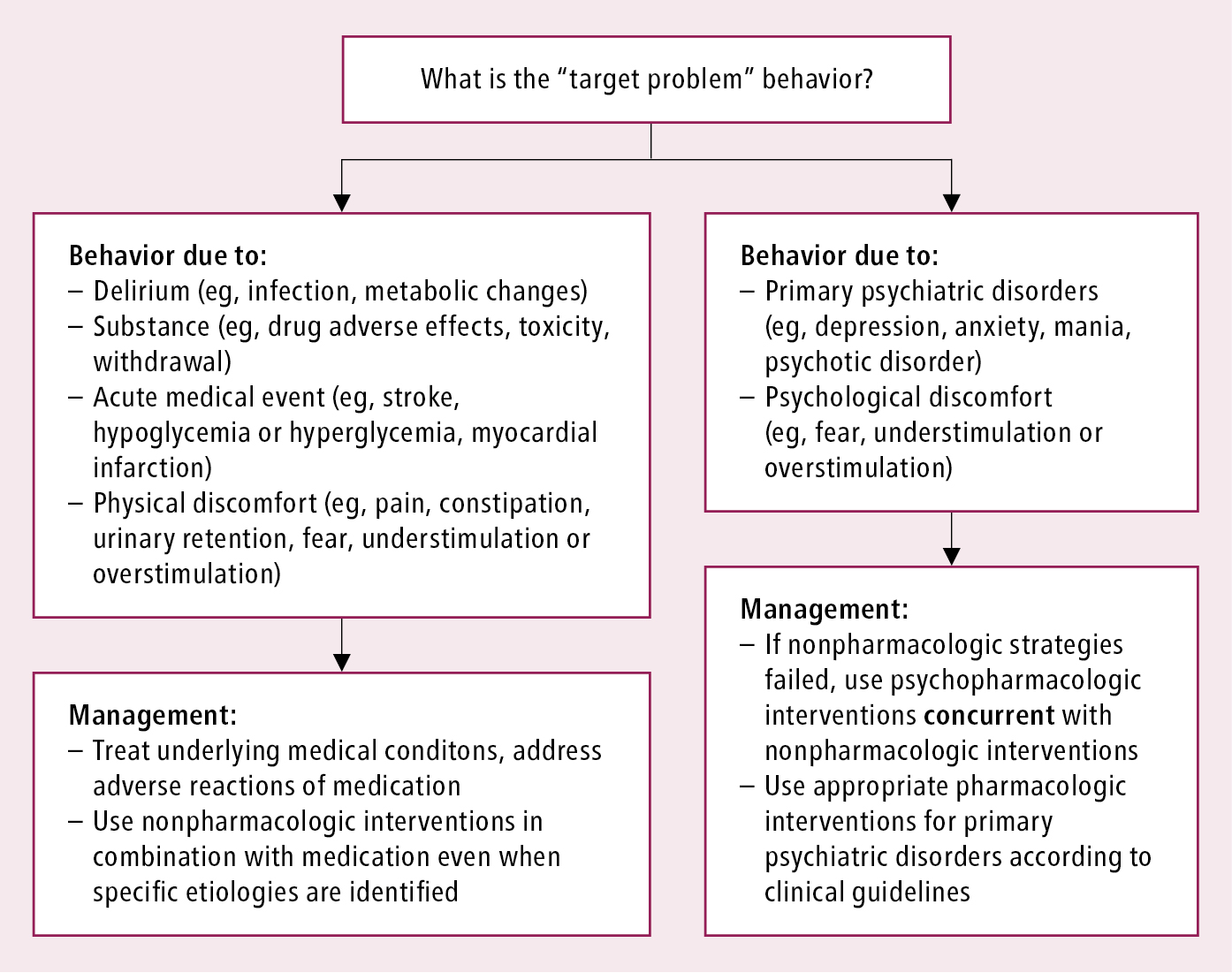
Figure 16.9-1. Identification of “target problem” behavior and its management.