Kittah NE, Vella A. Management of Endocrine Disease: Pathogenesis and management of hypoglycemia. Eur J Endocrinol. 2017 Jul;177(1):R37-R47. doi: 10.1530/EJE-16-1062. Epub 2017 Apr 5. PMID: 28381450.
Cryer PE, Axelrod L, Grossman AB, et al. Evaluation and Management of Adult Hypoglycemic Disorders: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2009 Mar;94(3):709-28. Epub 2008 Dec 16. doi: 10.1210/jc.2008-1410. PMID: 19088155.
Definition, Etiology, PathogenesisTop
Pathologic hypoglycemia is a state of low blood glucose concentration associated with signs or symptoms. The manifestations of hypoglycemia are nonspecific and may vary from person to person.
For this reason it is important to define pathologic hypoglycemia by the Whipple triad:
1) Signs and/or symptoms consistent with hypoglycemia.
2) Low plasma glucose concentration <3.0 mmol/L (55 mg/dL).
3) Quick resolution of signs and/or symptoms (within a few minutes) with an increase in blood glucose levels.
Diagnostic assessment should only be considered after Whipple triad is confirmed. In healthy individuals symptoms of hypoglycemia usually develop at a mean blood glucose concentration of 55 mg/dL (3.0 mmol/L). Symptoms can be attributed to the autonomic response to hypoglycemia, which includes sweating, weakness, palpitations, and tremor, or to neuroglycopenic effects, including irritability, confusion, visual disturbance, and seizure. Typical responses to hypoglycemia based on blood glucose concentration: Table 6.2-1.
Hypoglycemia occurs when the utilization of glucose by tissues (primarily the brain and muscle tissue) exceeds the supply (from digestion of carbohydrates and hepatic and renal glucose production). Glucose is the primary energy source for the brain and maintenance of adequate glucose concentrations in the bloodstream is essential to survival. Given that the brain cannot synthesize glucose or rely on alternative energy sources for prolonged periods, the body has several regulatory mechanisms to maintain appropriate glucose concentrations in the bloodstream. Key hormones involved in this balance include insulin and its counterregulatory hormone glucagon, epinephrine, cortisol, and growth hormone (GH). The first response to a falling glucose concentration is to reduce insulin secretion. Following this, glucagon secretion is stimulated as glucose concentrations continue to decline. If glucagon is deficient or the response to glucagon is inadequate, epinephrine secretion is stimulated to counteract hypoglycemia. GH and cortisol play a role in protracted hypoglycemia, but they appear to be less important in the acute phase. Due to the robust nature of these physiologic defense mechanisms, hypoglycemia in nondiabetic individuals is uncommon.
Causes of hypoglycemia can be further divided into those that affect ill or medicated patients and those that affect seemingly well individuals.
Also, it should be established whether hypoglycemia is fasting or postprandial. Fasting hypoglycemia usually occurs 4 to 5 hours after a meal, whereas postprandial hypoglycemia occurs much earlier, usually within 4 hours of a meal.
Causes of Hypoglycemia in Ill or Medicated Patients
1. Drugs: The most frequent cause of hypoglycemia, with hypoglycemic agents (insulin, sulfonylureas, or meglitinides) being the most common causative drugs (see Drug-Induced Hypoglycemia). Other culprits include alcohol, quinine (found in tonic water), disopyramide, certain fluoroquinolones, nonselective beta-blockers, high-dose salicylates, and pentamidine. Other nonantidiabetic agents associated with hypoglycemia: Table 6.2-2. The risk of developing drug-induced hypoglycemia increases with age, liver or renal disease, and polypharmacy.
2. Critical illness including sepsis, severe renal or hepatic failure, and less commonly cortisol deficiency. In these cases hypoglycemia is related to decreased glycogen stores, increased peripheral glucose utilization, or both.
3. Prescribing error: A particularly relevant cause of hypoglycemia in hospitalized patients (eg, failure of medication reconciliation, compromised dietary intake, frequent changing between hospital wards, inappropriate use of insulin sliding scale).
4. Hormone deficiencies: Hypoglycemia is related to low cortisol levels associated with primary adrenal insufficiency or less commonly with secondary or tertiary (central) adrenal insufficiency.
5. Non–islet cell tumors: Large non–islet cell tumors (often mesenchymal retroperitoneal tumors) can rarely cause hypoglycemia due to production of high-molecular-weight insulin-like growth factor 2 (IGF-2), which is structurally similar to insulin. They are characterized by hypoinsulinemia.
Causes of Hypoglycemia in Seemingly Well Patients
1. Insulinoma: A rare neuroendocrine tumor, most often in the pancreas, that causes inappropriate insulin secretion resulting in endogenous hyperinsulinism. It leads to spells of hypoglycemia, primarily in fasting states. Characteristic symptoms of spells include paroxysms of sweating, palpitations, blurred vision, and confusion and can also involve altered level of consciousness and convulsions. The vast majority of insulinomas are benign and insulinoma can be sporadic or associated with multiple endocrine neoplasia type 1 (MEN-1) syndrome.
2. Noninsulinoma pancreatogenous hypoglycemia syndrome (NIPHS) or nesidioblastosis: Characterized by spells of neuroglycopenia due to pancreatic beta-cell hypertrophy and dysfunction, driving endogenous hyperinsulinism. It typically causes postprandial hypoglycemia and is a rare syndrome with male predominance.
3. Post–gastric bypass hypoglycemia (PGBH): Hypoglycemia after bariatric surgery is most common among those who have undergone Roux-en-Y gastric bypass. It develops due to pancreatic beta-cell hypertrophy and dysfunction as well as an exaggerated incretin effect resulting in endogenous hyperinsulinism. It typically causes postprandial hypoglycemia, is more common in women than in men, and usually constitutes a late complication (ie, 6 months after surgery).
4. Autoimmune hypoglycemia: Autoantibodies to insulin cause hypoglycemia through inappropriate dissociation of insulin-antibody complexes, leading to hyperinsulinism and hypoglycemia. Conversely, autoantibodies to the insulin receptor cause hypoinsulinemic hypoglycemia by direct stimulation of insulin receptors. Autoimmune hypoglycemia usually causes postprandial hypoglycemia. Antibody formation can be triggered by certain drugs (eg, methimazole, captopril, diltiazem) and viruses. Autoimmune hypoglycemia can be associated with other autoimmune conditions.
5. Factitious hypoglycemia: It is caused by inappropriate use of hypoglycemic agents, such as insulin or oral insulin secretagogues. Factitious hypoglycemia can be accidental, surreptitious, or malicious and is more common among health-care professionals and those with family history of diabetes or psychiatric comorbidities.
DiagnosisTop
Diagnostic workup of hypoglycemia: Figure 6.2-1.
1. History and physical examination: Confirm the Whipple triad before considering investigations. Review the patient’s medication list for culprits bearing in mind that drugs are the most common cause of hypoglycemia. Assess the patient for recent surgeries (especially bariatric), infections, or major illnesses, which compromise the body’s physiologic regulatory mechanisms and increase susceptibility to hypoglycemia. Also, screen for current or previous psychiatric illness, access to diabetic medications, or employment in a health-care setting, which may point towards factitious or malicious hypoglycemia.
2. Blood tests: Blood tests should be evaluated during symptomatic episodes. Guided by history and low blood glucose levels on capillary testing or continuous glucose monitoring (CGM), measure the following parameters in venous blood: glucose, insulin, C-peptide, proinsulin, and beta-hydroxybutyrate (a ketone). If beta-hydroxybutyrate is elevated, consider testing blood alcohol levels to assess for alcohol-induced hypoglycemia. Interpretation of blood test results: Table 6.2-3. Measure blood concentrations of hypoglycemic medications, such as sulfonylurea and meglitinide, as indicated based on history. For autoimmune causes, levels of insulin and insulin receptor antibodies as well as results of additional tests (inflammatory markers [C-reactive protein, erythrocyte sedimentation rate] and antinuclear antibodies) may be informative. Consider testing for adrenal insufficiency, guided by history and physical examination. CGM may be helpful for appropriate timing of these tests.
3. Supervised 72-hour fast or modified overnight/daytime fast can be considered in the case of suspected fasting hypoglycemia and should be carried out in a supervised inpatient facility. The patient can consume noncaloric, caffeine-free beverages and should continue normal activity during testing. Nonessential medications should be withheld. Perform capillary blood glucose monitoring hourly until blood glucose concentration reaches 3.3 mmol/L. Then evaluate glucose concentration in venous blood. If it is ≤3.3 mmol/L or the patient develops neuroglycopenic symptoms, immediately measure venous glucose, insulin, C-peptide, proinsulin, and beta-hydroxybutyrate levels. In the absence of neuroglycopenic symptoms keep monitoring the above-listed parameters hourly until glucose drops to ≤2.8 mmol/L (50 mg/dL). If capillary blood glucose is <3.3 mmol/L and the patient has a decreased level of consciousness with hypoglycemia, after drawing the above-stated initial laboratory parameters, immediately give a single 50-mL dose of IV 50% dextrose in water (D50W). The test is considered positive when the patient develops neuroglycopenic symptoms of hypoglycemia and venous glucose drops to <3.3 mmol/L (60 mg/dL). Send samples for analysis only if glucose is <3.3 mmol/L (60 mg/dL). Insulin antibodies and levels of sulfonylureas or meglitinides should be measured as clinically indicated. At the end of the test, if hypoglycemia occurs, give 1 mg of IV glucagon and observe the response in glucose levels. Blood glucose concentrations should be measured 10, 20, and 30 minutes after glucagon administration. In hyperinsulinemic hypoglycemia (eg, due to insulinoma), glucagon should cause mobilization of glucose stores via glycogenolysis, yielding an increase in blood glucose by ≥1.4 mmol/L (25 mg/dL).
4. Mixed meal testing can be considered in postprandial hypoglycemia. Nonessential medications should be withheld. After an overnight fast the patients are given a nonliquid meal that typically provokes their symptoms (called a “mixed meal”). Then blood testing is performed to determine if they develop true hypoglycemia after eating. Check venous blood glucose, insulin, C-peptide, and proinsulin levels at baseline and every 30 minutes for 5 hours after the meal. Observe the patient for the development of symptoms or ask them to keep a written log. Send the above-mentioned samples for analysis only if glucose is <3.3 mmol/L (60 mg/dL). At the end of the test, if hypoglycemia develops, give 1 mg of IV glucagon and observe the response in blood glucose as above.
5. Evaluation for endogenous hyperinsulinism: Diagnosis is made by the presence of symptoms, signs, or both, with venous blood glucose <3.0 mmol/L (55 g/dL), insulin ≥21 pmol/L (3.0 microIU/mL), C-peptide ≥200 pmol/L (0.6 ng/mL), proinsulin ≥5 pmol/L, beta-hydroxybutyrate <2.7 mmol/L, and increase in blood glucose of ≥1.4 mmol/L (25 mg/dL) within 30 minutes after treatment with 1 mg of IV glucagon. If the above criteria are met, this indicates that hypoglycemia is mediated by endogenous insulin.
6. Imaging: If insulinoma is suspected, imaging is indicated only after biochemical confirmation of hyperinsulinemic hypoglycemia. Various modalities can be used, including contrast-enhanced abdominal computed tomography (CT), abdominal magnetic resonance imaging (MRI) with the pancreatic protocol, or endoscopic ultrasonography. In certain cases molecular/nuclear imaging with 68Ga-DOTATATE may be considered. The most sensitive method of localizing the mass is intraoperative ultrasonography at the time of resection.
7. Selective arterial calcium stimulation can be performed in specialized settings to distinguish between a localized insulinoma and more diffuse pancreatic islet cell hypertrophy in the context of hyperinsulinemic hypoglycemia. Testing is reserved for patients with biochemically confirmed hyperinsulinemic hypoglycemia in whom imaging is negative or inconclusive. The test involves injection of calcium gluconate (an insulin secretagogue) into the gastroduodenal, splenic, or superior mesenteric arteries with subsequent hepatic vein sampling for insulin to see the rise in insulin concentrations between the different arteries, which correlates with anatomic localization. A positive test is indicated by doubling or tripling of basal insulin concentrations. A significantly increased insulin concentration in the hepatic veins of a single artery compared with other hepatic veins is indicative of a localized lesion. Elevated insulin concentrations in multiple arteries suggest a diffuse process (ie, islet cell hypertrophy).
TreatmentTop
1. Acute hypoglycemia: Treatment depends on the severity of symptoms and the patient’s ability to tolerate oral intake. Do not delay treatment for confirmation of blood glucose levels given the low risk of adverse effects due to glucose administration. Options include:
1) 15 g of oral glucose (eg, orange juice, dextrose tablets).
2) 1 mg of IV or IM glucagon.
3) Single 3-mg dose of intranasal glucagon.
4) 50 mL (1 ampule) of IV 50% dextrose, followed by infusion of 10% dextrose at 100 mL/h to prevent recurrent episodes of hypoglycemia.
Patients with severe prolonged hypoglycemia may develop hypoglycemic coma due to cerebral edema. This is defined as reduced level of consciousness persisting for >30 minutes despite correction of hypoglycemia. Management includes 40 g of IV mannitol as a 20% solution over 20 minutes, glucocorticoids (eg, 10 mg of IV dexamethasone), or both.
2. Long-term management is based on addressing the underlying cause:
1) Drug-induced hypoglycemia: Avoid culprit medications or switch to alternatives. Be mindful of adjusting doses in renal or hepatic impairment to prevent hypoglycemia.
2) Insulinoma: The overall treatment is usually conducted in specialized settings. The mainstay of management is dietary modification, encouraging frequent low-carbohydrate meals. Surgical resection is definitive and the aim is for cure. Medical management can be considered in those with unresectable metastatic disease or those who are not surgical candidates. Diazoxide (given in divided doses [bid to tid] of 300-1200 mg/d) inhibits insulin secretion from beta cells. It is an effective treatment for preventing symptomatic hypoglycemia, but it is limited by adverse effects including edema, headache, insomnia, hirsutism, thrombocytopenia, and gastrointestinal (GI) upset. Somatostatin analogues such as octreotide (subcutaneous or IM) or lanreotide (deep subcutaneous administration only) can be used for refractory symptoms. Sometimes octreotide can also lead to unexpected hypoglycemia. Somatostatin analogues inhibit GH secretion at low doses and reduce insulin, glucagon, and thyroid-stimulating hormone (TSH) secretion at higher doses. Other medication options that could be tried empirically include oral verapamil, phenytoin, or glucagon.
The liver and regional lymph nodes are the most common sites of metastases. Management of metastatic disease is complex and should involve shared care provided by endocrinology, medical oncology, radiology, and endocrine surgery specialists. Certain advanced metastatic insulinomas can be managed with somatostatin analogues as above, radiolabeled octreotide or DOTATATE (peptide receptor radionuclide therapy [PRRT]), liver-directed hepatic artery embolization, or radiofrequency ablation. For advanced malignant tumors systemic chemotherapy or targeted biologic therapy may be considered. Everolimus, a mammalian target of rapamycin (mTOR) inhibitor, exerts its antitumor effect by inhibiting cell proliferation, angiogenesis, and metabolism. Adverse effects include rash, stomatitis, GI upset, and opportunistic infections. Tyrosine kinase inhibitors such as sunitinib can be used in advanced, well-differentiated tumors to inhibit angiogenesis within the tumor. They have multiple adverse effects including mucositis, rash, hepatitis, colitis, thyroid dysfunction, neutropenia, and hypoglycemia. Many of these therapies are usually considered after discussion in a multidisciplinary setting.
3) NIPHS or nesidioblastosis: Treatment depends on the severity of symptoms. Patients with mild to moderate symptom burden can benefit from lifestyle modification alone and oral acarbose at times. Patients should be counseled to limit their simple carbohydrate intake and have small, frequent meals. Somatostatin analogues may also be tried in severe disease. Those with more severe or debilitating symptoms may require partial or subtotal pancreatectomy.
4) Autoimmune hypoglycemia: Symptoms are generally self-limited and resolve with time. Consider discontinuation of the offending agent and treat the underlying cause if possible. If symptoms are persistent, consider treatment with acarbose, diazoxide, or systemic glucocorticoids; if they are refractory, consider using rituximab.
Special ConsiderationsTop
1. Driving: Hypoglycemic episodes carry safety concerns, especially with respect to operation of motorized vehicles for either personal or commercial purposes. Always ask patients whether they drive and follow your local regulations regarding driving and reporting hypoglycemia. Consult the local transport body if indicated. Advise patients to check their blood glucose levels every time prior to driving and at least every 4 hours during prolonged journeys. A useful way to remember the blood glucose target is “5 to drive,” meaning the blood glucose level should be ≥5.0 mmol/L prior to and during operation of a vehicle. Advise patients to keep snacks in the car along with a glucose monitoring device.
2. Other important considerations:
1) Recommend that the patient wear a medical alert bracelet or another identifier.
2) Educate the patient and/or family members on hypoglycemia symptom recognition and management.
3) Provide the patient with care by a multidisciplinary team including a dietitian, nurse educator, endocrinologist, or all of these.
Tables AND FIGURESTop
|
Blood glucose concentration (mmol/L) |
Typical symptoms |
|
<3.3 |
Sweating, anxiety, palpitations, tremor, hunger |
|
2.8–3.1 |
Cognitive dysfunction (confusion, mood change) |
|
2.5–2.8 |
Decreased level of consciousness |
|
<1.7 |
Coma |
|
<1.1 |
Convulsion, death |
|
Moderate QoE |
Low QoE |
Very low QoE |
|
– Cibenzoline – Gatifloxacin – Pentamidine – Quinine – Indomethacin – Glucagon (during endoscopy) |
– Chloroquineoxaline sulfonamide – Artesunate, artemisinin, artemether – IGF-1 – Lithium – Propoxyphene (dextropropoxyphene) |
– ACEIs – ARBs – Beta-adrenergic receptor antagonists – Levofloxacin – Disopyramide – TMP/SMX – Heparin – 6-mercaptopurine |
|
Adapted from Journal of Clinical Endocrinology & Metabolism, March 2009, 94(3): 709-728. | ||
|
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; IGF-1, insulin-like growth factor 1; QoE, quality of evidence; TMP/SMX, trimethoprim/sulfamethoxazole. | ||
|
Clinical state |
Signs/symptoms |
Insulina |
C-peptidea |
Proinsulina |
Beta-HBa |
Glucose response to glucagona |
OHA |
Autoantibodies |
|
Normal |
– |
< |
< |
< |
≥ |
< |
– |
– |
|
Excess exogenous insulin |
√ |
≥ |
< |
< |
< |
≥ |
– |
– |
|
Excess sulfonylurea/meglitinide |
√ |
≥ |
≥ |
≥ |
< |
≥ |
√ |
– |
|
Insulinoma, NIPHS, PGBH |
√ |
≥ |
≥ |
≥ |
< |
≥ |
– |
– |
|
Insulin autoimmune syndrome |
√ |
≥ |
≥ |
≥ |
< |
≥ |
– |
√ (to insulin) |
|
Insulin receptor autoimmune syndrome |
√ |
< |
< |
< |
< |
≥ |
– |
√ (to insulin receptor) |
|
IGF-2-secreting tumorb |
√ |
< |
< |
< |
< |
≥ |
– |
– |
|
a The symbols represent expected blood test results relative to the normal value or threshold as follows: – Insulin: 21 pmol/L or 3 microIU/mL. – C-peptide: 200 pmol/L or 0.6 ng/mL. – Proinsulin: 5 pmol/L. – Beta-HB: 2.7 mmol/L. – Glucose response to glucagon: 1.4 mmol/L or 25 mg/dL. b Additionally expect increased pro-IGF-2, free IGF-2, and IGF-2/IGF-1 ratio. | ||||||||
|
Adapted from Journal of Clinical Endocrinology & Metabolism, March 2009, 94(3): 709-728. | ||||||||
|
–, absent; √, present; beta-HB, beta-hydroxybutyrate; IGF, insulin-like growth factor; NIPHS, noninsulinoma pancreatogenous hypoglycemia syndrome; OHA, oral hypoglycemic agent; PGHB, post–gastric bypass hypoglycemia. | ||||||||
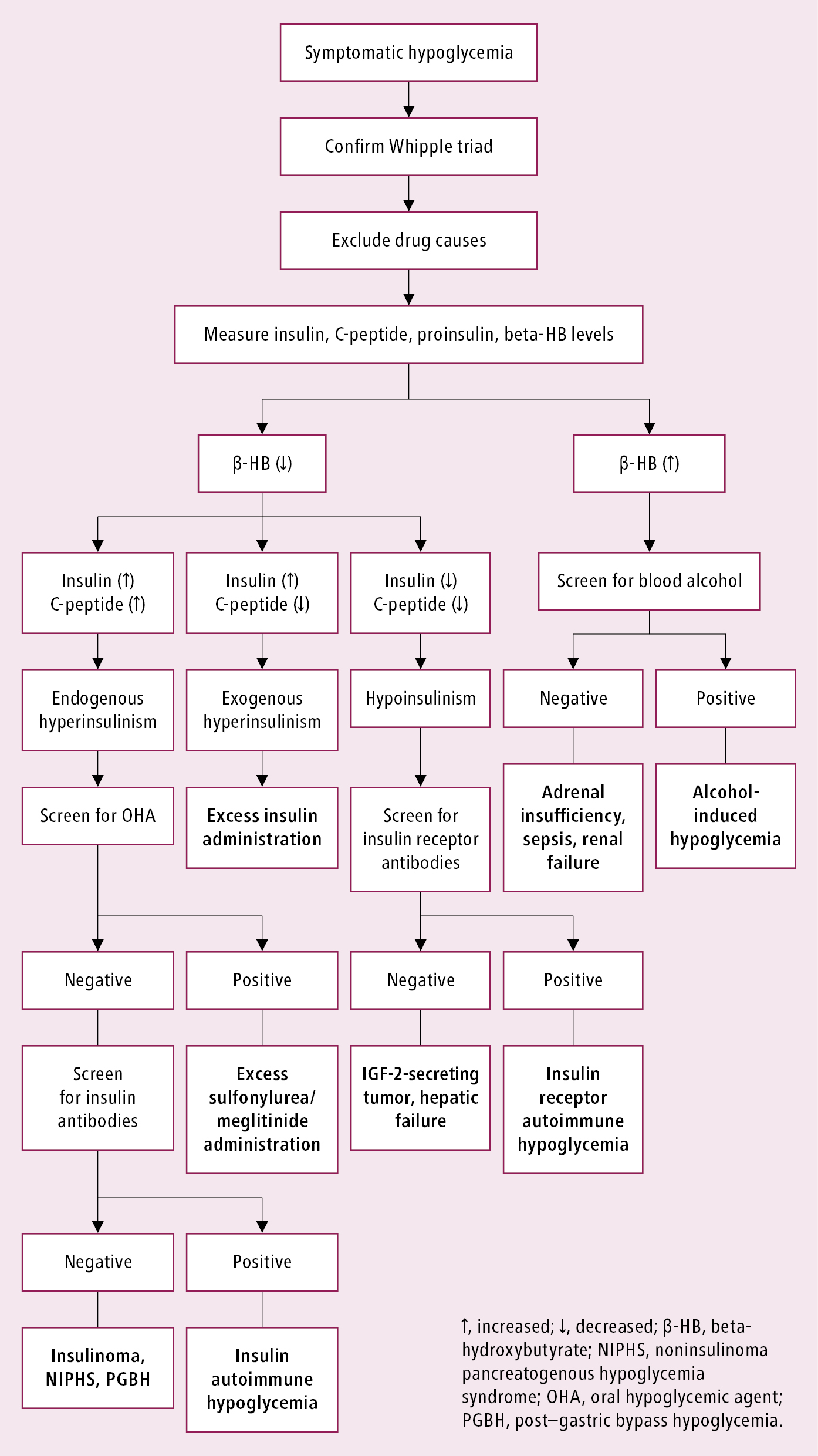
Figure 6.2-1. Diagnostic algorithm for hypoglycemia.