Bekkering GE, Agoritsas T, Lytvyn L, et al. Thyroid hormones treatment for subclinical hypothyroidism: a clinical practice guideline. BMJ. 2019 May 14;365:l2006. doi: 10.1136/bmj.l2006. PMID: 31088853.
Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid. 2017 Mar;27(3):315-389. doi: 10.1089/thy.2016.0457. Erratum in: Thyroid. 2017 Sep;27(9):1212. PMID: 28056690.
Pearce SH, Brabant G, Duntas LH, et al. 2013 ETA Guideline: Management of Subclinical Hypothyroidism. Eur Thyroid J. 2013 Dec;2(4):215-28. doi: 10.1159/000356507. Epub 2013 Nov 27. PMID: 24783053; PMCID: PMC3923601.
Garber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012 Nov-Dec;18(6):988-1028. Erratum in: Endocr Pract. 2013 Jan-Feb;19(1):175. PMID: 23246686.
Bensenor IM, Olmos RD, Lotufo PA. Hypothyroidism in the elderly: diagnosis and management. Clin Interv Aging. 2012;7:97-111. doi: 10.2147/CIA.S23966. Epub 2012 Apr 3. PMID: 22573936; PMCID: PMC3340110.
Abalovich M, Amino N, Barbour LA, et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2007 Aug;92(8 Suppl):S1-47. PMID: 17948378.
Definition, Etiology, PathogenesisTop
The clinical manifestations of hypothyroidism are caused by a deficiency of thyroxine (T4) and the resulting insufficient cellular effects of triiodothyronine (T3), leading to a general slowing down of metabolic processes and development of interstitial edema due to the deposition of fibronectin and hydrophilic glycosaminoglycans in the subcutaneous tissue, muscles, and other tissues.
The following types of hypothyroidism may be distinguished:
1) Primary hypothyroidism, which may be caused by:
a) Chronic autoimmune thyroiditis (most common cause; also known as Hashimoto thyroiditis) and other types of subacute or acute thyroiditis (painful or painless).
b) Total or subtotal thyroidectomy (with a possible autoimmune process leading to insufficiency of the remaining thyroid parenchyma).
c) Treatment with 131I (radioactive iodine).
d) Irradiation of the neck.
e) Excessive intake of iodides, including the administration of amiodarone and iodine contrast media (by inhibiting iodide organification and T4 and T3 synthesis—known as the Wolff-Chaikoff effect—which could be transient or result in permanent hypothyroidism in cases of failure to escape the acute effect).
f) Excess of antithyroid drugs (transient and reversible hypothyroidism that improves upon discontinuation of the drug or dose reduction).
g) Administration of lithium salts (inhibiting T4 and T3 secretion), phenytoin, some tyrosine kinase inhibitors (eg, sunitinib, sorafenib), interferon alpha, or interleukin 2.
h) Significant environmental iodine deficiency and chronic exposure to goitrogens, that is, substances that inhibit the accumulation of iodides by the thyroid gland (eg, perchlorates, thiocyanates, nitrates).
i) Infiltrative diseases (eg, hemochromatosis, sarcoidosis, amyloidosis).
j) Congenital hypothyroidism (eg, thyroid agenesis, dysgenesis, inborn errors of metabolism, or defects in hormone synthesis).
k) Immunotherapy-associated autoimmune thyroiditis.
2) Central hypothyroidism, which results from a decrease in or lack of thyroid-stimulating hormone (TSH; secondary hypothyroidism) or thyrotropin-releasing hormone (TRH; tertiary hypothyroidism) secretion caused by pituitary or hypothalamic disease, respectively:
a) Sellar masses/neoplasia.
b) Inflammatory, infectious, or infiltrative conditions.
c) Vascular, traumatic, or iatrogenic injury (radiation, neurosurgery procedures).
d) Hemorrhagic necrosis (Sheehan syndrome).
e) Immunotherapy-associated autoimmune hypophysitis.
f) Other forms of hypophysitis.
3) Thyroid hormone resistance (very rare).
Clinical Features and Natural HistoryTop
In secondary (pituitary-related) and tertiary (hypothalamus-related) hypothyroidism the signs and symptoms are usually less pronounced than in primary hypothyroidism, but there may be coexisting features of other endocrine deficiencies (look for the features of adrenal insufficiency, diabetes insipidus/vasopressin deficiency, or other symptoms directly related to hypopituitarism). Primary hypothyroidism can also be a part of polyglandular autoimmune syndromes.
Subclinical hypothyroidism (SH) is defined as an elevated TSH but normal free thyroxine (FT4). In patients with SH signs and symptoms of hypothyroidism are typically absent (~70%). Fatigue, memory and mood impairments, elevated serum total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C), and raised triglyceride levels may be present. Approximately 2% to 5% of patients per year progress to overt hypothyroidism. This risk increases 2-fold if an elevated TSH level is accompanied by high titers of thyroperoxidase antibodies (TPOAb) or if the TSH level is consistently >10 mIU/L. The nonspecific symptoms of both subclinical and overt hypothyroidism make the clinical distinction difficult.
1. General signs and symptoms: Weight gain, fatigue, weakness and poor exercise tolerance, somnolence, general slowing down (affecting both psychomotor functions and speech), cold intolerance.
2. Cutaneous manifestations: Dry, cold, pale, and/or slightly yellowish skin; reduced perspiration; excessive epidermal keratosis; subcutaneous edema (so-called myxedema) with coarse facial features; edema of the eyelids and hands; brittle hair; thinning of hair and eyebrows.
3. Cardiovascular manifestations: Bradycardia, hypokinetic pulse, distant heart sounds; enlarged cardiac silhouette; hypertension (diastolic) and hypotension in extreme cases. Pericardial effusions may also be present in severe cases.
4. Respiratory manifestations: Hoarse and mellow voice (due to thickened vocal cords and an enlarged tongue), shallow breathing and a low respiratory rate, chronic signs and symptoms of upper respiratory tract congestion, in severe cases symptoms of respiratory failure. Pleural effusions may also be present in severe cases.
5. Gastrointestinal (GI) manifestations: Chronic constipation, ileus (in severe cases), ascites (in advanced disease; usually accompanied by pericardial and pleural effusions).
6. Renal manifestations: Impaired water excretion (potentially leading to volume overload and hyponatremia). In the absence of clinically overt edema the abnormalities are likely insignificant.
7. Nervous system manifestations: Cognitive dysfunction, nerve entrapment syndromes (eg, carpal tunnel syndrome), paresthesias, hyporeflexia, delayed relaxation phase of deep tendon reflexes, hearing impairment.
8. Musculoskeletal manifestations: Muscle weakness and fatigue, loss of energy, lethargy, muscle cramps, myalgia; joint edema, particularly affecting the knees (due to synovial hypertrophy and effusion).
9. Reproductive system manifestations: In women, menstrual dysfunction (reduced cycle duration, menorrhagia), infertility, miscarriage, and galactorrhea; in men, loss of libido and erectile dysfunction.
10. Psychiatric manifestations: Impaired concentration and memory, subclinical or overt depression, mood lability, bipolar disorder, or paranoid psychosis. In severe cases dementia and coma.
Myxedema coma is a life-threatening condition that develops in extremely severe untreated hypothyroidism.
Symptoms include hypothermia (body temperature <30 degrees Celsius), bradycardia, hypotension, hypoxemia and hypercapnia with respiratory acidosis (caused by hypoventilation), hypoglycemia, hyponatremia with symptoms of heart failure (HF)/volume overload, edema, cognitive dysfunction, coma, and shock. Patients may have reduced muscle tone, hyporeflexia, and can rarely develop seizures.
Triggers of myxedema coma include nonadherence with levothyroxine (L-T4) therapy, infection, myocardial infarction, stroke, GI bleeding, surgery, trauma, and medications such as amiodarone, lithium, anesthetics, narcotics, beta-blockers, diuretics, phenytoin, and barbiturates.
DiagnosisTop
1. Hormone tests:
1) Serum TSH concentrations: Elevated in primary hypothyroidism (the key diagnostic criterion), low or not appropriately elevated in central hypothyroidism.
2) Low serum FT4 levels.
3) Serum free triiodothyronine (FT3) levels may often be normal, or sometimes low, and are thus often not useful.
2. Other laboratory tests:
1) Elevated antithyroid antibody levels (primarily TPOAb) in autoimmune thyroid disease.
2) Elevated levels of TC, LDL-C, and triglycerides.
3) Anemia (normocytic or macrocytic).
4) Hyponatremia.
5) Elevated creatine kinase (CK).
1. Primary hypothyroidism:
1) Overt: Low serum FT4 and elevated serum TSH levels.
2) Subclinical: Normal serum FT4 and elevated serum TSH levels.
2. Central hypothyroidism: Low serum FT4 with normal or low serum TSH levels.
3. Myxedema coma: Low serum FT4 and usually markedly elevated serum TSH levels. The diagnosis depends primarily on clinical manifestations, precipitating factors, and exclusion of other causes of coma. Clinical hallmarks include hypothermia, cardiac dysfunction, respiratory dysfunction, and decreased level of consciousness.
Differential diagnostic algorithm for hypothyroidism: Figure 6.7-1. When considering the etiologies of primary hypothyroidism, look for a family history of thyroid diseases and/or other autoimmune diseases, exposure to iodine or chemical goitrogens, recent childbirth, treatment with antithyroid drugs, immunotherapy, previous thyroid surgery, 131I therapy, or neck irradiation (including treatment completed several years earlier).
Autoimmune hypothyroidism may be accompanied by insufficiency of other endocrine glands or other autoimmune diseases. In the case of central hypothyroidism, look for coexisting adrenal insufficiency before initiating hormone replacement therapy.
Edema and effusions in body cavities must be differentiated from chronic kidney disease, nephrotic syndrome, liver disease, and HF.
In patients hospitalized with an acute illness or in those receiving certain medications (eg, dopamine, glucocorticoids), serum TSH levels may be decreased. Predominantly as a result of impaired synthesis, the levels of thyroid hormone–binding proteins also decrease in nonthyroidal illness, which leads to low serum total T3 and T4 levels. FT4 levels may also be found to be low, although methods for assessing FT4 are unreliable during severe illness. Reverse T3 (rT3) levels increase and FT3 levels decrease. The term euthyroid sick syndrome or nonthyroidal illness is used to describe these laboratory abnormalities. Despite such abnormal findings, patients usually do not warrant thyroid hormone replacement therapy. In view of the abnormal results of hormone tests of the pituitary-thyroid axis observed in hospitalized patients with severe illness, thyroid function tests should be performed only if there is a strong suspicion of clinically relevant thyroid dysfunction; if the results are abnormal, thyroid testing should be repeated when the patient improves in a few weeks or as clinically indicated.
TreatmentTop
Overt hypothyroidism is an indication for hormone replacement therapy, usually lifelong.
Long-Term Hormone Replacement Therapy
Monotherapy with L-T4 is the treatment of choice.
Dosage: The daily dose is estimated on an individual basis. In adults it is usually ~1.6 microg/kg/d; in older patients or in patients with cardiovascular disease (CVD), use lower doses, starting at 25 to 50 microg daily. In young healthy adults initiating treatment with full replacement doses is suggested.Evidence 1Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). Moderate Quality of Evidence (moderate confidence that we know true effects of intervention). Quality of Evidence lowered due to imprecision (a low number of events). Roos A, Linn-Rasker SP, van Domburg RT, Tijssen JP, Berghout A. The starting dose of levothyroxine in primary hypothyroidism treatment: a prospective, randomized, double-blind trial. Arch Intern Med. 2005 Aug 8-22;165(15):1714-20. Erratum in: Arch Intern Med. 2005 Oct 24;165(19):2227. PMID: 16087818. Dose adjustments are guided by measuring the TSH level 4 to 6 weeks following the initiation and again 4 to 6 weeks after L-T4 dose changes. L-T4 should be titrated to target a normal TSH level.
L-T4 should be taken 1 hour before a meal, at bedtime, or 3 to 4 hours after a meal. Some drugs may reduce the absorption or metabolism of L-T4 (eg, calcium or iron supplements, bisphosphonates, sulfonylureas, proton pump inhibitors [PPIs]) and thus should be taken 3 to 4 hours apart from L-T4.
Fixed combinations of L-T4 and liothyronine (L-T3) are not recommended, as T4 is converted to T3. L-T3 is used rarely, mostly for rapid correction of the thyroid hormone deficit in myxedema coma.
In regions with adequate iodine intake, iodine supplementation should not be used in the treatment of hypothyroidism, except in pregnant women at risk for iodine deficiency (endemic areas).
In the case of coexisting adrenal insufficiency, treating hypothyroidism without glucocorticoid therapy leads to acute adrenal crisis due to increased metabolism of cortisol and lower free cortisol levels. Therefore, in patients with coexisting hypothyroidism and adrenal insufficiency, always replace glucocorticoids first (see Primary Adrenal Insufficiency).
In SH treatment with L-T4 is suggested for patients with serum TSH levels >10 mIU/L or, more recently, even >20 mIU/L in the elderly.Evidence 2Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). Low Quality of Evidence (low confidence that we know true effects of the intervention). Quality of Evidence lowered due to indirectness and the observational nature of data. Bekkering GE, Agoritsas T, Lytvyn L, et al. Thyroid hormones treatment for subclinical hypothyroidism: a clinical practice guideline. BMJ. 2019 May 14;365:l2006. doi: 10.1136/bmj.l2006. PMID: 31088853. Rodondi N, den Elzen WP, Bauer DC, et al; Thyroid Studies Collaboration. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010 Sep 22;304(12):1365-74. doi: 10.1001/jama.2010.1361. PMID: 20858880; PMCID: PMC3923470. For patients with a TSH between the upper reference limit (URL) and 10 mIU/L, L-T4 treatment has shown no appreciable effect on quality of life or thyroid-related symptoms. However, a trial of treatment can be considered in symptomatic patients. In SH treatment is targeted to normalize the TSH level. Small doses of L-T4 (25-75 microg) are often adequate. In the elderly there is really no benefit of treating SH unless the patient is truly symptomatic and the TSH is >10 to 20 mIU/L.
Treatment monitoring: For primary hypothyroidism assess serum TSH levels 4 to 6 weeks after the last L-T4 dose adjustment. Once TSH normalizes, repeat the measurement every 6 to 12 months or earlier if there are new clinical manifestations, pregnancy, significant changes in health or weight, or any potential drug interactions. Watch for iatrogenic thyrotoxicosis, as this can lead to adverse events such as atrial fibrillation (AF) and osteoporosis.
In patients with central hypothyroidism, measure the FT4 level and aim for a level in the mid-upper range of normal (TSH is not useful in such cases).
Increasing the previously established L-T4 replacement dose may be necessary in the following cases:
1) Oral L-T4 malabsorption (eg, inflammatory bowel disease, celiac disease, bariatric surgery, atrophic gastritis).
2) Concomitant administration of drugs that cause L-T4 malabsorption or maldigestion (eg, cholestyramine, aluminum hydroxide, calcium and iron supplements, atrophic gastritis, gut surgery, PPIs).
3) Starting estrogen products (eg, oral contraceptives).
4) Using drugs that increase L-T4 metabolism (phenobarbital, phenytoin).
5) Weight gain.
6) Pregnancy.
Discontinuation or tapering of L-T4 may be appropriate in the following cases, while serially monitoring thyroid indices, since recovery may occur:
1) Painful subacute thyroiditis.
2) Painless subacute thyroiditis, if thyroid autoantibodies are low or absent.
3) SH, if no symptomatic improvement is seen in patients with a TSH <10 to 20 mIU/L.
4) In patients under palliative care nearing the end of life, to decrease pill burden.
Myxedema coma is an endocrine emergency associated with a high mortality risk (>20%) that should be treated aggressively.
1. L-T4 alone: On day 1 administer 200 to 400 microg IV in a single infusion or using an infusion pump (to correct FT4 deficiency; this may result in evident clinical improvement within several hours). If there is no response in 24 hours, addition of L-T3 is suggested (see below). On subsequent days administer 100 microg of IV L-T4 for 1 day in a rapid infusion or using an infusion pump (take special precautions in patients with coronary heart disease due to the high risk of triggering angina, HF, or arrhythmia and in older patients); then use 50 microg IV daily. When improvement is seen, switch to oral L-T4 administration (it is not necessary to start with a low dose of L-T4 and titrate it up).
2. Alternatively, IV L-T4 and L-T3 may be used: On day 1 administer 200 to 300 microg of IV L-T4 (4 microg/kg lean body weight) plus a separate L-T3 preparation (5-20 microg IV infusion); on subsequent days administer 1.6 microg/kg/d of IV L-T4 plus 2.5 to 10 microg of IV L-T3 tid for 1 to 2 days or until the patient improves or is alert (use lower doses in the elderly and in patients at high risk of cardiovascular complications).
If IV preparations are not available, oral combinations containing 20 microg of L-T3 and 100 microg of L-T4 may be used: on day 1 administer 3 to 4 tablets once daily via a nasogastric tube; on subsequent days, 1 or 2 tablets via a nasogastric tube. When the patient’s condition improves, administer 1 tablet once daily or L-T4 100 to 150 microg/d. Note that oral treatment is less reliable than the IV route due to the possibility of malabsorption.
3. Ensure good ventilation: Intubation and ventilatory support are usually required.
4. Correct any electrolyte disturbances and hypoglycemia using IV fluids; do not use hypotonic fluids due to the risk of water intoxication. In cases of major hyponatremia (<125 mmol/L) or seizures, consider a hypertonic NaCl infusion (see Hyponatremia). In euvolemic patients with hyponatremia treatment is the same as in patients with the syndrome of inappropriate antidiuresis (SIAD) (see Syndrome of Inappropriate Antidiuresis (SIAD)).
5. Aggressively treat concomitant diseases, such as HF or infection (administer empiric antibiotic therapy until culture and susceptibility results are available). Always look for and treat the precipitant.
6. Until the possibility of concomitant adrenal insufficiency is excluded, administer glucocorticoids in stress doses (eg, IV hydrocortisone 50-100 mg every 6-8 h). Glucocorticoids may be discontinued immediately after adrenal insufficiency has been excluded in a sample collected before the administration of hydrocortisone.
7. Do not actively rewarm a hypothermic patient, as this may cause vascular dilation and shock (a warm blanket is sufficient to prevent further heat loss).
8. Ensure drugs are dosed carefully due to the risk of decreased metabolism in this condition.
Special ConsiderationsTop
1. During pregnancy thyroxine-binding globulin and T4 concentrations increase by week 7 of gestation and peak by week 16. In the first trimester human chorionic gonadotropin (hCG) stimulates the TSH receptor, increasing thyroid hormone production and reducing TSH levels. There is no fetal production of T4 until the second trimester.
2. The most common cause of hypothyroidism in pregnancy, when iodine nutrition is adequate, is autoimmune thyroid disease. Adverse outcomes associated with maternal hypothyroidism include premature birth, low birth weight, placenta abruption, miscarriage, and lower IQ.
3. According to the 2017 American Thyroid Association guidelines, screening for thyroid disorders before planned pregnancy is indicated in women at high risk of thyroid disease, which includes:
1) History of thyroid disorders, thyroidectomy, goiter, postpartum thyroiditis, type 1 diabetes mellitus or other autoimmune disorders, miscarriage or preterm delivery, therapeutic head or neck irradiation.
2) Age >30 years.
3) Family history of thyroid disease.
4) Elevated plasma levels of TPOAb.
5) Signs or symptoms suggestive of thyroid disorders.
6) Infertility.
7) Morbid obesity (body mass index [BMI] >40 kg/m2).
8) Use of amiodarone or lithium.
4. In pregnant women the TSH level is usually measured at weeks 4 to 8 of pregnancy, during the first obstetric visit. According to the American Thyroid Association (2017), if a population-specific and trimester-specific reference range for serum TSH is not available, an URL of ~4 mIU/L may be used. For most assays this limit represents a reduction in the URL for TSH in nonpregnant patients by ~0.5 mIU/L.
5. Measure TPOAb levels in:
1) Patients with a TSH level >2.5 mIU/L, if it alters management and prompts L-T4 treatment.
2) Patients with a positive history of postpartum thyroiditis.
3) Patients who are treated for infertility or have a history of miscarriage or preterm delivery. Significant correlations between elevated TPOAb and miscarriage, preterm delivery, and neonatal respiratory failure have been identified.
6. Diagnostic criteria:
1) Overt hypothyroidism: A TSH level above the upper limit of the trimester-specific reference range and low FT4 level (local TSH range for the first trimester, 0.1-2.5 mIU/L; second trimester; 0.2-3.0 mIU/L; third trimester, 0.3-3.0 mIU/L).
2) SH: A TSH level above the upper limit of the trimester-specific reference range and normal FT4 level.
7. Treatment:
1) Target therapy towards normalization of the trimester-specific TSH (between the lower reference limit and 2.5 mIU/L).
2) In pregnant patients with overt hypothyroidism start L-T4 at a dose that covers the daily requirement. According to current European guidelines, the dose of L-T4 in overt hypothyroidism in pregnancy is 2 to 2.33 microg/kg/d (with TSH >10 mIU/L). In patients diagnosed with hypothyroidism before pregnancy, increase the dose of L-T4 by 20% to 30% upon confirmation of pregnancy. Patients can simply administer 2 additional tablets weekly to their current L-T4 dosage. Post partum L-T4 should be reduced to the preconception dose and TSH level should be then retested in 6 weeks.
3) In pregnant women with SH, L-T4 is recommended for TPO-positive women if TSH is greater than the pregnancy-specific reference range (or, if the range is unavailable, >4 mIU/L) and suggested for TPO-negative women if TSH is >4 mIU/L.Evidence 3Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). Note: There is a disagreement among authors regarding the strength of this recommendation (strong vs weak). Low Quality of Evidence (low confidence that we know true effects of the intervention). Quality of Evidence lowered due to indirectness and the nonrandomized nature of data. Negro R, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Stagnaro-Green A. Universal screening versus case finding for detection and treatment of thyroid hormonal dysfunction during pregnancy. J Clin Endocrinol Metab. 2010 Apr;95(4):1699-707. doi: 10.1210/jc.2009-2009. Epub 2010 Feb 3. PMID: 20130074. Reid SM, Middleton P, Cossich MC, Crowther CA, Bain E. Interventions for clinical and subclinical hypothyroidism pre-pregnancy and during pregnancy. Cochrane Database Syst Rev. 2013 May 31;5:CD007752. doi: 10.1002/14651858.CD007752.pub3. Review. PMID: 23728666. In SH the suggested starting dose of L-T4 is 1.2 microg/kg/d with TSH <4.2 mIU/L, and 1.42 microg/kg/d when TSH is between 4.2 and 10 mIU/L.
4) Supplementation with potassium iodide is provided in many prenatal vitamins in North America and is especially relevant in geographic areas where access to iodine is of concern.
5) TSH should be checked every 4 weeks in the first half of pregnancy (up to 20 weeks) and then every trimester thereafter. Recheck TSH 4 to 6 weeks after any dose change.
1. In people aged >60 years the signs of hypothyroidism can be mild and nonspecific. Symptoms may include cognitive and memory disturbances, depression, anemia, HF, hypercholesterolemia, constipation, balance problems, and joint and muscle pains. In the event of hyponatremia or elevated serum CK in the elderly, it is important to exclude underlying hypothyroidism. In the event of elevated TSH, exclude convalescence after severe illness (TSH is usually ≤20 mIU/L).
2. Diagnosis: TSH levels rise physiologically with aging, independent of any accompanying thyroid conditions. Therefore, in persons >60 years of age it is proposed to consider higher thresholds of TSH: 6 mIU/L at the age of 60 to 70 years, and 8 mIU/L in persons >70 years.
3. Treatment:
1) Overt hypothyroidism: Thyroid replacement therapy is indicated in elderly patients.
2) SH: An elevated serum TSH should be confirmed by repeating the measurement in 3 to 6 months before initiating treatment. The decision to treat depends on several factors including age, comorbidities, frailty, symptoms, and TSH level. In fit elderly patients with a TSH level >10 mIU/L, thyroid replacement is recommended. In frail elderly patients monitoring alone can be attempted (measure TSH every 3-6 months), as these patients are more vulnerable to L-T4 adverse effects. If TSH levels are ≤10 mIU/L we do not recommend treating SH.
Follow the general principles of treating hypothyroidism, but start treatment at 25 to 50 microg/d and increase by increments of 25 microg after 6 to 8 weeks in accordance with the patient’s symptoms. North American guidelines suggest targeting a TSH of 4 to 6 mIU/L in patients aged >70 years, if treated.
4. The most frequent complications of treatment in the elderly are myocardial ischemia and arrhythmias (especially AF).
FiguresTop
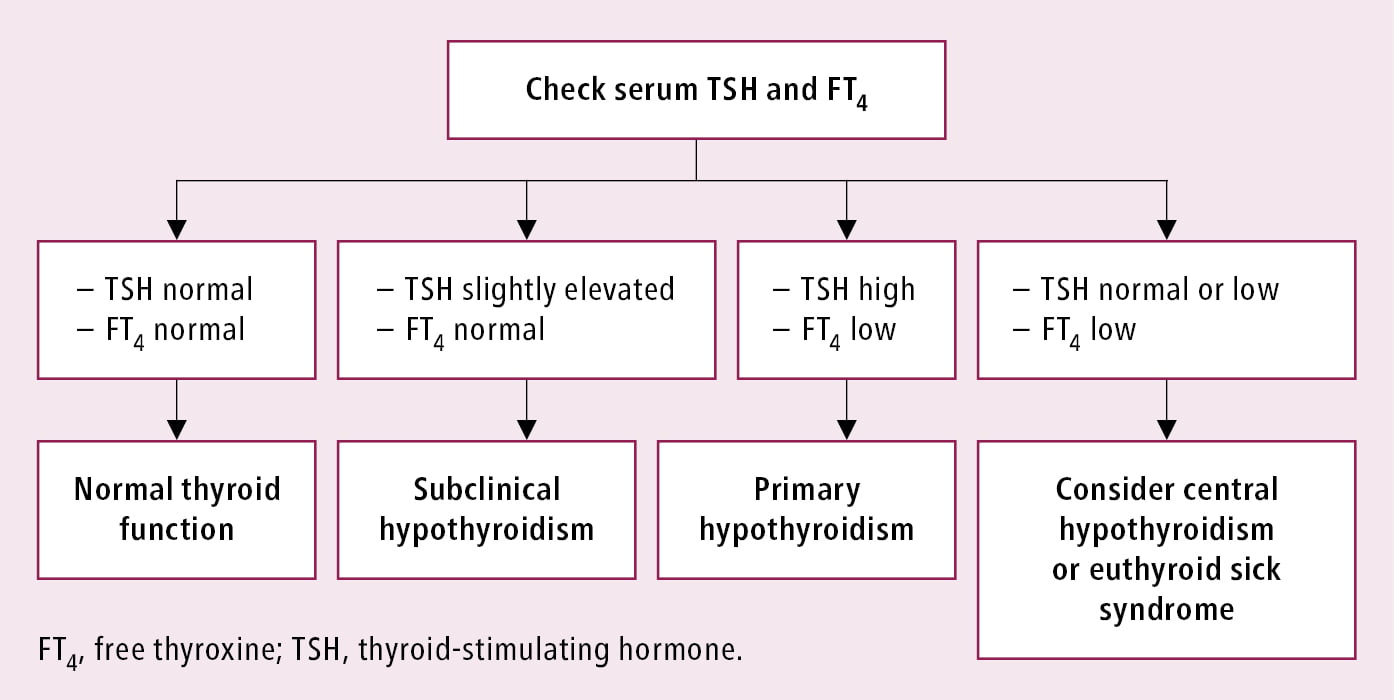
Figure 6.7-1. Diagnostic algorithm of hypothyroidism based on TSH and FT4 levels.