de Silva D, Singh S, Muraro A, et al; European Academy of Allergy and Clinical Immunology Food Allergy and Anaphylaxis Guidelines Group. Diagnosing, managing and preventing anaphylaxis: Systematic review. Allergy. 2021 May;76(5):1493-1506. doi: 10.1111/all.14580. Epub 2020 Sep 29. PMID: 32880997.
Cardona V, Ansotegui IJ, Ebisawa M, et al. World Allergy Organization Anaphylaxis Guidance 2020. World Allergy Organ J. 2020 Oct 30;13(10):100472. doi: 10.1016/j.waojou.2020.100472. PMID: 33204386; PMCID: PMC7607509.
Shaker MS, Wallace DV, Golden DBK, et al. Anaphylaxis-a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020 Apr;145(4):1082-1123. doi: 10.1016/j.jaci.2020.01.017. Epub 2020 Jan 28. PMID: 32001253.
Simons FE, Ardusso LR, Bilò MB, et al. International consensus on (ICON) anaphylaxis. World Allergy Organ J. 2014 May 30;7(1):9. doi: 10.1186/1939-4551-7-9. eCollection 2014. PMID: 24920969; PMCID: PMC4038846.
Dhami S, Panesar SS, Roberts G, et al; EAACI Food Allergy and Anaphylaxis Guidelines Group. Management of anaphylaxis: a systematic review. Allergy. 2014 Feb;69(2):168-75. doi: 10.1111/all.12318. Epub 2013 Nov 20. PMID: 24251536.
Definition, Etiology, PathogenesisTop
Anaphylaxis is a severe, life-threatening, generalized or systemic rapid-onset hypersensitivity reaction (allergic or nonallergic).
Anaphylactic shock is a severe rapidly progressing anaphylactic reaction (anaphylaxis) resulting in a life-threatening drop in blood pressure.
Operational definition of anaphylaxis: Table 2.2-1.
The most frequent causes of anaphylaxis:
1) Allergic:
a) Drugs: Most commonly beta-lactam antibiotics, paralytic drugs, barbiturates, biologic drugs, and cytotoxic agents.
b) Hymenoptera venoms (honeybee, bumblebee, yellow jacket, hornet, fire ant).
c) Proteins administered via parenteral routes, including blood and its products, enzymes (eg, streptokinase), sera (eg, tetanus immunoglobulin), allergens used for in vivo diagnosis and immunotherapy.
d) Foods: In adults most commonly fish, seafood, peanuts, cow’s milk, chicken egg, and mammalian meat proteins (delayed anaphylaxis may occur 3-6 hours after consumption of mammalian meat products, eg, beef or pork).
e) Inhaled allergens, for instance, animal dander.
f) Latex.
g) Dialysis membranes sterilized with ethylene oxide.
h) Vaccines.
i) Disinfectants from the biguanide group (eg, chlorhexidine).
2) Nonallergic:
a) Direct release of mediators from mast cells: Opioids, muscle relaxants, colloids (eg, dextran, hydroxyethyl starch, human albumin), hypertonic solutions (eg, mannitol), physical exercise, low temperature.
b) Immunologic complexes: Blood and its products, immunoglobulins, animal sera, vaccines, dialysis membranes.
c) Alterations of arachidonic acid metabolism: Hypersensitivity to acetylsalicylic acid (ASA) and other nonsteroidal anti-inflammatory drugs (NSAIDs).
d) Histamine and tyramine present in foods (this makes an anaphylactic reaction more severe).
e) Other mechanisms: Radiologic contrast media, food contaminants and preservatives, or unknown causes.
Nonallergic reactions are not mediated by immunologic mechanisms, and therefore shock may occur even at the first exposure to a certain factor. In ~30% of cases the causative factor remains unknown despite a thorough diagnostic workup (idiopathic anaphylaxis). However, the most frequent mechanism of anaphylaxis is an IgE-related reaction, while nonimmune reactions are less prevalent. Their common feature is degranulation of mast cells and basophils. The released mediators, such as histamine, tryptase, arachidonic acid metabolites, platelet activating factor, or nitric oxide, cause bronchial and gastrointestinal (GI) smooth muscle contraction and vasodilation, increased vascular permeability, stimulation of sensory nerve endings, recruitment of inflammatory cells, and activation of the complement, coagulation, and fibrinolysis cascades. Increased vascular permeability and rapid fluid transfer to extravascular compartment may lead to the loss of as much as 35% of effective circulating blood volume within ~10 minutes.
Risk factors for anaphylaxis include a previous episode of anaphylaxis and re-exposure to the triggering factor (beta-lactam antibiotics, hymenoptera venoms, radiographic contrast agents), adult age, female sex (anaphylaxis is more common and severe in women than in men), atopy, allergen entry site (after administration of the antigen parenterally, especially IV, reactions are frequent and severe), mastocytosis, mast cell and basophil activation syndromes, simultaneous exposure to the allergen administered parenterally and occurring in the environment (eg, allergen immunotherapy during the pollen season), medical procedures (eg, administration of diagnostic agents, in vivo tests, provocation tests, surgery under local or general anesthesia).
The most common causes of anaphylaxis in adults are drugs (~35%), food (~30%), and insect venoms (20%), while in children, food (70%), insect venoms (20%), and medications (7%). In ~30% of adults and ~15% of children, the cause of anaphylaxis (idiopathic) cannot be identified. Occasionally anaphylaxis requires ≥2 factors (eg, exposure to the sensitizing allergen and exercise) to be close in time for anaphylaxis to occur.
Clinical Features and Natural HistoryTop
Signs and symptoms of anaphylaxis most often occur within seconds or minutes of exposure to a trigger (although in some cases they may develop later, even after several hours):
1) Skin and subcutaneous tissue: Urticaria/angioedema and erythema occur in up to 90% of patients.
2) Respiratory system: Upper airway edema, hoarseness, stridor, cough, wheezing, respiratory compromise, rhinitis.
3) GI system: Nausea, vomiting, abdominal pain, diarrhea.
4) Systemic reactions: Hypotension and other symptoms of shock occur in 30% of patients; they may occur simultaneously with other signs and symptoms of anaphylaxis or (usually) develop shortly after their onset.
5) Less frequent: Dizziness or headache, uterine cramps, anxiety, feeling of impending doom.
The more rapidly the symptoms develop, the higher the risk of severe and life-threatening anaphylaxis. However, it should be noted that symptoms that are initially mild (eg, limited to the skin and subcutaneous tissue) may also rapidly become life-threatening if adequate treatment is not started promptly. Delayed or biphasic reactions may occur, with symptoms developing or relapsing over 3 to 8 hours. Symptoms of anaphylaxis may last for hours or even days despite adequate treatment.
Signs and symptoms of anaphylactic shock (regardless of trigger) include cold and pale skin, sweating, collapsed subcutaneous veins, hypotension, tachycardia, oliguria or anuria, loss of bowel control, and loss of consciousness. Circulatory arrest may occur.
DiagnosisTop
According to the 2011 World Allergy Organization guidelines, anaphylaxis is highly likely when any of the following criteria is fulfilled:
1) Acute onset of illness (minutes to several hours) with involvement of the skin, mucosal tissue, or both (eg, urticaria, itching, lips-tongue-uvula edema), which is accompanied by either (or both):
a) Respiratory compromise (eg, dyspnea, stridor, wheeze-bronchospasm, hypoxemia).
b) Reduced blood pressure or associated symptoms of end-organ dysfunction (eg, hypotonia, syncope, incontinence).
2) Exposure to a likely allergen for a patient is rapidly followed by ≥2 of the following (within minutes to several hours):
a) Involvement of the skin-mucosal tissue (eg, urticaria, itching, lips-tongue-uvula edema).
b) Respiratory compromise (eg, dyspnea, stridor, wheeze-bronchospasm, hypoxemia).
c) Reduced blood pressure or associated symptoms (eg, hypotonia, syncope, incontinence).
d) Persistent GI symptoms (crampy abdominal pain, vomiting).
3) Decreased blood pressure following exposure to a “known allergen” (within minutes to several hours):
a) Infants and children: Low systolic pressure (age-related) or a decrease >30% in systolic blood pressure (in children low systolic blood pressure is defined as <70 mm Hg from 1 month to 1 year; <[70 mm Hg + 2 × age] from 1-10 years; and <90 mm Hg from 11-17 years).
b) Adults: A systolic blood pressure <90 mm Hg or >30% compared with baseline.
Infants are more likely to have respiratory compromise than hypotension or shock. In this age group, shock is more likely to be manifested initially by tachycardia than by hypotension.
1. Assays measuring levels of histamine or methylhistamine are not widely available, not specific for anaphylaxis, and not routinely used.
2. Assaying total tryptase levels has become more common. Tryptase should be measured within 2 hours of the onset of symptoms and then repeated 24 hours after the symptoms have resolved to get a basal level. The minimal elevation in the acute total tryptase level that is considered to be clinically significant has been suggested as ≥2 + 1.2 × baseline tryptase levels. However, tryptase may not be elevated in a third of patients and its usefulness in excluding diagnosis is thus limited, if any.Evidence 1High Quality of Evidence (high confidence that we know true effects of the intervention). Sala-Cunill A, Cardona V, Labrador-Horrillo M, et al. Usefulness and limitations of sequential serum tryptase for the diagnosis of anaphylaxis in 102 patients. Int Arch Allergy Immunol. 2013;160(2):192-9. doi: 10.1159/000339749. Epub 2012 Sep 25. PMID: 23018683.
3. Where there is a clear causative agent, it is best to simply avoid exposure rather than confirm the cause by performing a challenge test. Often sensitivity may be lost over time and when alternative agents cannot be used, cautious challenge under close observation and after a full discussion with the patient could be considered. Such an attempt to establish the allergic cause of anaphylaxis with skin tests should be made, if considered essential, no earlier than 3 to 4 weeks after an episode. Generally, challenge tests are not recommended after anaphylactic shock. Assessing the levels of IgE specific to suspected allergens may be helpful.
TreatmentTop
1. Assess airway, breathing, circulation (ABC), and level of consciousness. Establish and maintain the airway if necessary. In case of respiratory or cardiac arrest, start cardiopulmonary resuscitation (see Cardiac Arrest). In patients with stridor or significant edema of the face and upper airways (tongue, oral and throat mucosa, hoarseness), consider immediate endotracheal intubation. If delayed, it may be progressively more difficult, and unsuccessful intubation attempts may further aggravate edema. If edema causes life-threatening airway obstruction and attempts of endotracheal intubation have been unsuccessful, perform cricothyroidotomy.
2. Stop exposure to the suspected antigen (eg, stop drug infusion or blood transfusion).
3. Call for help.
4. Administer epinephrine as soon as possible once anaphylaxis is recognized (or even suspected):
1) In patients with a history of anaphylaxis who carry an epinephrine autoinjector, immediately administer 1 dose of IM epinephrine in the lateral thigh in case of even minor symptoms suggestive of anaphylaxis.
2) In patients without cardiac arrest, administer IM epinephrine in the lateral thigh in a dose of 0.01 mg/kg of a 1:1000 (1 mg/mL) solution up to a maximum of 0.5 mg in adults (0.3 mg in children weighing >25 kg).
Record the time of injection and repeat it in 5 to 15 minutes as necessary. Most patients respond to 1 to 2 doses. Patients who do not respond to an IM injection of epinephrine and fluid resuscitation (shock is imminent or has already developed) should receive epinephrine in a slow IV infusion; the dose should be titrated on the basis of the effect of treatment on blood pressure assessed using continuous noninvasive monitoring. In adults the starting dose is 1 to 10 microg/min in an IV infusion (0.1 mg/mL; 1:10,000 solution contains 100 microg/mL or 1000 microg in a 10-mL syringe). Do not use IV epinephrine bolus (unless required during cardiac arrest).
5. Place the patient on the back, or in a different position of comfort and safety if there is respiratory distress or vomiting, and elevate the lower extremities, as this may be helpful in the management of hypotension. Do not let the patient sit or stand up suddenly or be placed in an upright position.
6. Administer oxygen 6 to 8 L/min via a face mask (see Oxygen Therapy). This is indicated in patients in whom it was necessary to administer several doses of epinephrine, patients with respiratory distress, signs and symptoms of myocardial ischemia, or chronic diseases of the respiratory system.
7. Establish peripheral IV access with 2 large-bore needles (optimally ≥1.6-1.8 mm [16-14 gauge]) and use infusion kits allowing for rapid fluid administration.
8. Administer IV fluids: In patients with a substantial decrease in blood pressure who do not respond to IM epinephrine, administer a rapid IV infusion of 1 to 2 L of isotonic crystalloid (eg, in adults 5-10 mL/kg of 0.9% saline over the first 5-10 minutes [10 mL/kg in children]).
9. Monitor blood pressure and, depending on the clinical situation, also electrocardiography (ECG), oxygen saturation (SpO2), or arterial blood gases.
1. In patients receiving beta-blockers who do not respond to epinephrine, consider administration of IV glucagon 1 to 2 mg in a slow infusion over ~5 minutes and then in a continuous IV infusion 5 to 15 microg/min, depending on the clinical response. Nausea, vomiting, and hyperglycemia are frequent adverse effects.
2. IV H1 antihistamines are recommended in case of itching, urticaria, angioedema, and nasal and ocular symptoms (diphenhydramine 25-50 mg [1 mg/kg; maximum, 50 mg in children]). H1-receptor blockers do not prevent or relieve upper airway obstruction, hypotension, or shock, and they should never be substituted for epinephrine.Evidence 2Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). Low Quality of Evidence (low confidence that we know true effects of the intervention). Quality of Evidence lowered due to the lack of direct experimental data in humans. At the same time, this treatment is routinely used based on the use of this class of drugs in less severe allergic conditions and we recommend it unless better data become available. Sheikh A, ten Broek Vm, Brown SG, Simons FE. H1-antihistamines for the treatment of anaphylaxis with and without shock. Cochrane Database Syst Rev. 2007 Jan 24;(1):CD006160. Review. PMID: 17253584. The dose could be repeated or doubled.
3. Consider an IV H2 antagonist in case of hypotension: ranitidine 50 mg every 8 to 12 hours or 150 mg orally bid.Evidence 3Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). Low Quality of Evidence (low confidence that we know true effects of the intervention). Quality of Evidence lowered due to only indirect data from other allergic conditions where H2-antagonists improved some outcomes when given in conjunction with an H1 antagonist. Lin RY, Curry A, Pesola GR, et al. Improved outcomes in patients with acute allergic syndromes who are treated with combined H1 and H2 antagonists. Ann Emerg Med. 2000 Nov;36(5):462-8. PMID: 11054200. Lorenz W, Duda D, Dick W, et al. Incidence and clinical importance of perioperative histamine release: randomised study of volume loading and antihistamines after induction of anaesthesia. Trial Group Mainz/Marburg. Lancet. 1994 Apr 16;343(8903):933-40. PMID: 7512679. A double dose may be used.
4. Give an IV glucocorticoid,Evidence 4Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due to only indirect evidence from other acute allergic conditions (acute asthma) and no direct experimental data available in humans. Consequently, the dose is not clear. Choo KJ, Simons FE, Sheikh A. Glucocorticoids for the treatment of anaphylaxis. Cochrane Database Syst Rev. 2012 Apr 18;4:CD007596. doi: 10.1002/14651858.CD007596.pub3. Review. PMID: 22513951. Smith M, Iqbal S, Elliott TM, Everard M, Rowe BH. Corticosteroids for hospitalised children with acute asthma. Cochrane Database Syst Rev. 2003;(2):CD002886. Review. PMID: 12804441. Rowe BH, Spooner C, Ducharme FM, Bretzlaff JA, Bota GW. Early emergency department treatment of acute asthma with systemic corticosteroids. Cochrane Database Syst Rev. 2001;(1):CD002178. Review. PMID: 11279756 eg, methylprednisolone 1 to 2 mg/kg, then 1 mg/kg/d, or hydrocortisone 200 to 400 mg, then 100 mg every 6 hours, for a maximum of 3 days. Although this is not effective in treatment of the acute phase of anaphylactic shock, it may prevent the late phase of anaphylaxis. As in asthma, if there is wheezing, glucocorticoids may potentiate the beta-agonist receptors. In the case of anaphylaxis without signs and symptoms of shock, airway edema, or respiratory compromise, an oral glucocorticoid (eg, prednisone 0.5 mg/kg/d) may be used. Do not use it as a substitute for epinephrine or as first-line treatment.
5. Inhaled bronchodilators should be used if wheezing, coughing, and shortness of breath are not relieved by epinephrine. Preferably use nebulized albuterol (INN salbutamol) 2.5 or 5 mg in 3 mL 0.9% saline or 2 to 4 puffs with the AeroChamber if the patient is able to follow instructions. Inhalation may be repeated when necessary. Bronchodilators reduce airway obstruction but do not prevent or relieve laryngeal symptoms, hypotension, or shock and should never be used instead of epinephrine.
6. In the case of subglottic laryngeal edema, administer epinephrine in oxygen nebulization as a single dose of 1 mg in 4 mL of 0.9% NaCl. The dose can be repeated as needed.
7. Admit the patient to an intensive care unit without delay if anaphylaxis is refractory to treatment.
Follow-UpTop
Follow-up after the resolution of signs and symptoms of anaphylaxis:
1) Monitor the patients for 8 to 24 hours for a possible late-phase reaction or protracted anaphylaxis. Extend the monitoring to 24 hours in patients with severe anaphylaxis of unknown etiology, slow onset of symptoms, severe asthma, or severe bronchospasm, or if continued exposure to the triggering allergen is probable, as well as in patients with a history of biphasic anaphylactic reactions.
2) If symptoms of anaphylaxis do not recur within 8 hours of completed treatment, the patient may be discharged. They should be advised of the possibility of recurrent symptoms and instructed how to act in such situations. The patient should receive a prescription for an epinephrine autoinjector, written anaphylaxis emergency action plan, and medical identification (bracelet, necklace) indicating that they have a history of anaphylaxis and specifying the responsible agent (if known). Educate the patient and family or caregivers on the usage of epinephrine autoinjector and emergency action plan. The patient may also receive a prescription for an oral nonsedating antihistamine with advice to take it after an epinephrine injection, especially in the presence of skin lesions in the course of anaphylaxis and if oral intake is possible.
3) Refer the patient to an allergy specialist to confirm the diagnosis and suspected triggers and to plan prevention strategies and further management (see below).
PreventionTop
In patients with a history of suspected anaphylaxis or an episode diagnosed as anaphylaxis, establish whether it was actually anaphylaxis and whether the suspected trigger was a true trigger of the reaction. Investigations of the putative trigger should be performed not earlier than 3 to 4 weeks after the episode. Initial evaluation and management in patients with a history of suspected anaphylaxis: Figure 2.2-1.
1. Measures used to reduce the risk of anaphylactic shock:
1) Administration of drugs: If possible, administer drugs orally rather than parenterally. Always ask about allergies when taking history, particularly prior to IV drug administration. Never ignore notes added by other physicians or the patients’ opinion on drug hypersensitivity. Follow the recommended testing for hypersensitivity and administration of the drug that may trigger anaphylaxis. If the drug is administered IM or subcutaneously, make sure that the needle has not been introduced into a blood vessel. Monitor the patient for 30 to 60 minutes after the administration of a drug that may potentially induce anaphylaxis.
2) Administration of vaccines or sera:
a) Antiviral vaccines: Take a history on hypersensitivity to egg proteins (influenza, yellow fever; influenza vaccine could be still administered in a setting where a severe allergic reaction can be treated) and polyethylene glycol (PEG) (mRNA coronavirus disease 2019 [COVID-19] vaccines). There are rare case reports of PEG anaphylaxis and other reactions to IM injections.
b) Antitoxins (eg, tetanus, diphtheria, botulinum toxoids, snake venom serum): Administer human serum. If human serum is not available and allergy to animal serum is suspected, administer both H1-receptor and H2-receptor blockers and an oral or IV glucocorticoid prior to serum administration.
3) Allergy diagnostic testing: Whenever possible, skin prick tests should be performed before proceeding to intradermal tests. Since intradermal tests are about 1000 times as potent as skin prick tests, if the skin prick test is negative, use a 1:100 dilution of the reagent used for skin prick testing to perform an intradermal test, which is then 10 times as potent as the skin prick test. Intradermal tests are used for diagnosis of anaphylaxis to venoms and drugs such as penicillin. For pollen-allergic patients, skin prick tests are usually sensitive enough and can be performed at any time. Challenge tests with drugs, either oral or bronchial, should be performed in an inpatient or outpatient setting with emergency medications and equipment to promptly treat anaphylaxis under direct medical supervision by a clinician with an appropriate training and immediate access to supportive care, if needed.
4) Allergy testing post anaphylaxis: see above.
2. Medical procedures associated with an increased risk of anaphylaxis (eg, specific allergen immunotherapy, IV biologic drugs, or contrast media):
1) Equipment and drugs: Stethoscope, blood pressure measurement device; tourniquets, syringes, hypodermic needles, large-bore needles (16-14 gauge); injectable epinephrine (1 mg/mL); equipment for oxygen therapy (see Oxygen Therapy); airway masks and a self-inflating (Ambu) ventilation bag with a facial mask; 0.9% saline (at least 500 mL bottles or bags) and IV transfusion kits; IV antihistamines: both H1 antagonists and H2 antagonists (eg, ranitidine); IV glucocorticoids (eg, methylprednisolone, hydrocortisone); nebulizer and inhaled short-acting beta-agonist (eg, salbutamol).
2) The risk associated with administration of an allergen, drug, or diagnostic agent may be reduced by previous administration of oral H1-receptor blockers (eg, cetirizine, rupatadine, bilastine, or desloratadine) and H2-receptor blockers (eg, ranitidine) and/or a glucocorticoid (eg, prednisone 0.5 mg/kg orally 3 times: 12, 7, and 1 hour before the administration of a drug or diagnostic agent that may induce anaphylaxis).
Secondary prevention refers to prevention methods in patients with a history of anaphylactic shock. Adequate use of prophylactic measures requires appropriate patient education.
1. Avoidance of triggers, if identified.
2. Carrying an epinephrine autoinjector at all times.
3. Carrying a relevant medical identification bracelet, necklace, or card.
4. Pharmacologic prophylaxis: Long-term antihistamine treatment in patients with idiopathic anaphylaxis, or prophylactic administration of H1 and H2 antihistamines and/or a glucocorticoid prior to expected exposure to a trigger (eg, before contrast-enhanced imaging [see above]). This is not effective in exercise-induced anaphylaxis or in most forms of IgE-dependent anaphylaxis.
5. Specific allergen immunotherapy, if possible (eg, specific immunotherapy in patients with allergy to hymenoptera venom or specific immunotherapy for a drug allergy), or immune tolerance induction (in patients with hypersensitivity to drugs, eg, chemotherapeutic agents, monoclonal antibodies, antibiotics, ASA).
1) Oral desensitization to a specific food, such as milk, eggs, or peanuts, can be achieved in carefully selected patients monitored by physicians.Evidence 5Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). High Quality of Evidence (high confidence that we know true effects of the intervention). Jones SM, Pons L, Roberts JL, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009 Aug;124(2):292-300, 300.e1-97. doi: 10.1016/j.jaci.2009.05.022. Epub 2009 Jul 3. PMID: 19577283; PMCID: PMC2725434. Hofmann AM, Scurlock AM, Jones SM, et al. Safety of a peanut oral immunotherapy protocol in children with peanut allergy. J Allergy Clin Immunol. 2009 Aug;124(2):286-91, 291.e1-6. doi: 10.1016/j.jaci.2009.03.045. Epub 2009 May 27. PMID: 19477496; PMCID: PMC2731305. Adverse effects, sometimes requiring the administration of epinephrine, occur during oral desensitization.
2) An anti-IgE antibody given subcutaneously can increase the margin of protection against inadvertently ingested foods (and other allergens) for patients at risk of anaphylaxis.Evidence 6Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due to indirectness of outcome measures. An anti-IgE antibody given subcutaneously can increase the margin of protection against inadvertently ingested foods (and other allergens) for patients at risk of anaphylaxis, although it is not a cure. Leung DY, Sampson HA, Yunginger JW, et al; Avon Longitudinal Study of Parents and Children Study Team. Effect of anti-IgE therapy in patients with peanut allergy. N Engl J Med. 2003 Mar 13;348(11):986-93. Epub 2003 Mar 10. PMID: 12637608. This is still an experimental strategy.
3) For anaphylaxis triggered by a medication, if it is not possible to substitute for an alternative drug, physician-supervised desensitization strategies with the offending agent are effective and safe, particularly for beta-lactam or other antibiotics, ASA, other NSAIDs, and chemotherapy agents.Evidence 7Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due to the observational nature of data, but results are consistent and therefore confidence was increased to moderate. Celik W, Pichler WJ, Adkinson NF Jr. Drug allergy. In: Adkinson NF Jr., Bochner BS, Busse WW, Holgate ST, Lemanske RF Jr., Simons FER, eds. Middleton’s Allergy: Principles and Practice. 7th ed. St Louis: Mosby, Inc.; 2009: 1205-26. Castells MC, Tennant NM, Sloane DE, et al. Hypersensitivity reactions to chemotherapy: outcomes and safety of rapid desensitization in 413 cases. J Allergy Clin Immunol. 2008 Sep;122(3):574-80. doi: 10.1016/j.jaci.2008.02.044. Epub 2008 May 27. PMID: 18502492. Mirakian R, Leech SC, Krishna MT, et al; Standards of Care Committee of the British Society for Allergy and Clinical Immunology. Management of allergy to penicillins and other beta-lactams. Clin Exp Allergy. 2015 Feb;45(2):300-27. doi: 10.1111/cea.12468. PMID: 25623506. Lee RU, White AA, Ding D, et al. Use of intranasal ketorolac and modified oral aspirin challenge for desensitization of aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol. 2010 Aug;105(2):130-5. doi: 10.1016/j.anai.2010.05.020. Epub 2010 Jul 1. PMID: 20674823. Wong JT, Nagy CS, Krinzman SJ, Maclean JA, Bloch KJ. Rapid oral challenge-desensitization for patients with aspirin-related urticaria-angioedema. J Allergy Clin Immunol. 2000 May;105(5):997-1001. PMID: 10808182. Desensitization lasts as long as the medication is regularly administered. However, immunologic tolerance does not occur, and if the medication is discontinued for a time, symptoms recur when it is restarted.
4) Stinging insect venom anaphylaxis can be prevented by desensitization in most patients who receive a 3-year to 5-year course of subcutaneous immunotherapy to the relevant venom.Evidence 8Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of the intervention). Freeman TM. Clinical practice. Hypersensitivity to hymenoptera stings. N Engl J Med. 2004 Nov 4;351(19):1978-84. Review. PMID: 15525723. Golden DB. Insect sting allergy and venom immunotherapy: a model and a mystery. J Allergy Clin Immunol. 2005 Mar;115(3):439-47; quiz 448. Erratum in: J Allergy Clin Immunol. 2005 Jun;115(6):1128. PMID: 15753884.
Tables and FiguresTop
|
Anaphylaxis is highly likely when any of the following 2 criteria is fulfilled: |
|
1. Acute onset of illness (minutes to several hours) with simultaneous involvement of the skin, mucosal tissue, or both (eg, generalized hives, pruritus or flushing, swollen lips-tongue-uvula), and ≥1 of the following: – Respiratory compromise (eg, dyspnea, wheeze-bronchospasm, stridor, reduced PEF, hypoxemia) – Reduced BP or associated symptoms of end-organ dysfunction (eg, hypotonia [collapse], syncope, incontinence) – Severe GI symptoms (eg, severe crampy abdominal pain, repetitive vomiting), especially after exposure to nonfood allergens |
|
2. Acute onset of hypotensiona or bronchospasm or laryngeal involvement after exposure to a known or highly probable allergen for that patient (minutes to several hours), even in the absence of typical skin involvement |
|
a Hypotension in adults is defined as a decrease in systolic BP >30% from baseline or <90 mm Hg. |
|
Adapted from World Allergy Organ J. 2020 Oct 30;13(10):100472. |
|
BP, blood pressure; GI, gastrointestinal; PEF, peak expiratory flow. |
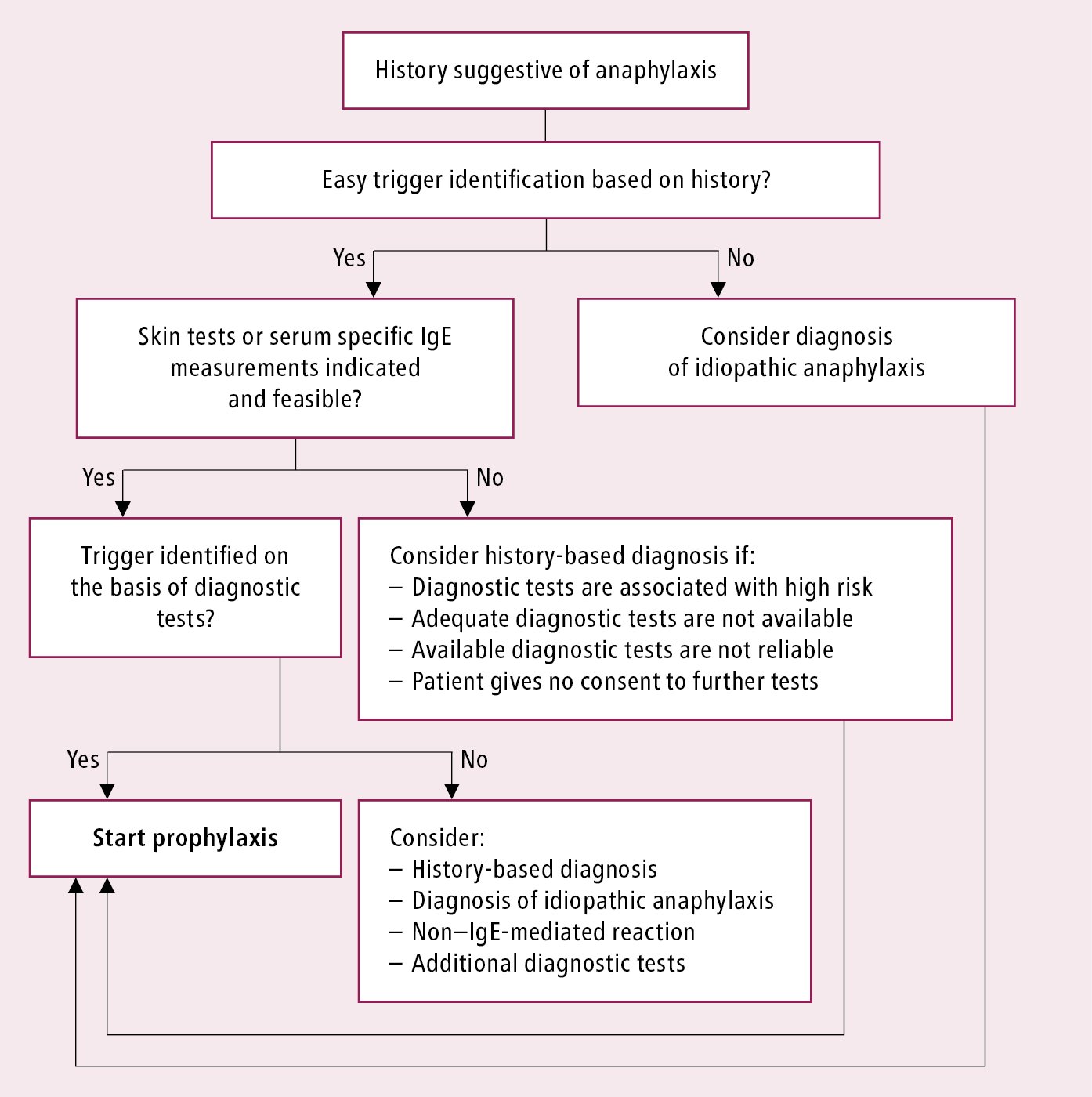
Figure 2.2-1. Algorithm of initial evaluation and management in patients with suspected anaphylaxis. Adapted from Ann Allergy Asthma Immunol. 2015;115(5):341-84.