Wanhainen A, Van Herzeele I, Bastos Goncalves F, et al. Editor's Choice -- European Society for Vascular Surgery (ESVS) 2024 Clinical Practice Guidelines on the Management of Abdominal Aorto-Iliac Artery Aneurysms. Eur J Vasc Endovasc Surg. 2024;67(2):192-331. doi:10.1016/j.ejvs.2023.11.002
Isselbacher EM, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022;146(24):e334-e482. doi:10.1161/CIR.0000000000001106
Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018 Jan;67(1):2-77.e2. doi: 10.1016/j.jvs.2017.10.044. PMID: 29268916.
Erbel R, Aboyans V, Boileau C, et al; ESC Committee for Practice Guidelines. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014 Nov 1;35(41):2873-926. doi: 10.1093/eurheartj/ehu281. Epub 2014 Aug 29. Erratum in: Eur Heart J. 2015 Nov 1;36(41):2779. PMID: 25173340.
Definition, Etiology, PathogenesisTop
Aortic aneurysm is a local dilation of the aorta by >50% of its normal diameter. Normal aortic dimensions in adults: Table 3.19-1.
Anatomy of the aorta: Figure 3.19-1.
Classifications of aortic aneurysms:
1) Based on etiology: Atherosclerotic aneurysm (most common by far), degenerative aneurysm (Marfan syndrome, Ehlers-Danlos syndrome type IV, cystic degeneration of the aorta), postinflammatory aneurysm (Takayasu disease, giant cell arteritis, inflammation in the course of systemic diseases, syphilis, infective endocarditis, sepsis), posttraumatic aneurysm.
2) Based on shape: Fusiform aneurysm (much more frequent), saccular aneurysm (Figure 3.19-2).
3) Based on wall structure: True aneurysm, pseudoaneurysm (or “false aneurysm”; after disruption of the intima and media, the aneurysm wall is formed by the adventitia together with surrounding tissues; this type is often posttraumatic; Figure 3.19-2).
4) Based on location: Thoracic aneurysm (usually of the ascending aorta but also can be seen in the aortic arch or descending thoracic aorta), abdominal aneurysm (located below the diaphragm; infrarenal aneurysms constitute ~90% of aortic aneurysms), thoracoabdominal aneurysm (more extensive, involving both the thoracic and abdominal aorta).
5) Based on clinical presentation: Asymptomatic, symptomatic, dissecting (Figure 3.19-2), ruptured.
Clinical Features and Natural HistoryTop
What follows below pertains to an aneurysm that is either asymptomatic or causing symptoms related to the space it occupies. It does not correspond to acute medical emergency of aortic dissection or aneurysm rupture (see Complications, below).
The majority of aortic aneurysms, regardless of location, are asymptomatic and most commonly identified as an incidental finding on imaging. The first manifestation of an aortic aneurysm may be a thromboembolic incident, such as stroke (in the case of ascending or aortic arch aneurysm), lower limb ischemia, intestinal ischemia, renal infarction, or blue toe syndrome (acute ischemia [sometimes necrosis] of the toes caused by small emboli originating from the mural thrombus that may line the inner surface of the aneurysm [Figure 3.19-3]).
1. Symptoms and signs of a thoracic aortic aneurysm: Chest pain and back pain (in 25% of patients without dissection; usually persistent, stabbing, often severe), dysphagia (rare), hoarseness, cough, dyspnea (sometimes dependent on body position), hemoptysis, recurrent pneumonia, and Horner syndrome. Aneurysms of the ascending aorta or aortic arch may cause symptoms of aortic regurgitation (often with manifestations of heart failure) or symptoms of superior vena cava syndrome.
2. Symptoms and signs of an abdominal aortic aneurysm: Symptoms are usually absent. The most frequent manifestation is a constant dull pain that may be abdominal, hypogastric, or lumbar and mimics radicular pain (movement has no effect on pain intensity; may be less severe in the supine position with bent knees). Aneurysms ≥5 cm in diameter may be palpable if examined for. They are frequently tender, especially when expanding rapidly. Murmurs may be audible over the abdominal aorta.
3. Natural history: Aneurysms have a tendency to expand and rupture (see Complications, below). The 5-year risk of rupture of an abdominal aortic aneurysm is estimated at 2% for aneurysms <40 mm in diameter, 20% for aneurysms >50 mm in diameter, and 40% for aneurysms >60 mm in diameter. An increase in aneurysm diameter by 5 mm over 6 months is associated with a 2-fold increase in the risk of rupture.
Ascending thoracic aortic aneurysms expand by an average of 1 mm per year (aneurysms of the descending aorta, large aneurysms, and those associated with Marfan syndrome grow faster; in patients with Loeys-Dietz syndrome they may expand >10 mm per year). The 1-year risk of rupture is 7% for aneurysms >60 mm in diameter and 2% for aneurysms <50 mm in diameter.
Aortic aneurysms may undergo dissection, although this happens rarely, as preexisting aneurysms generally do not dissect but rather expand, rupture, or embolize. Conversely, subacute or chronic aortic dissections may proceed to secondary aneurysmal dilation and potentially associated complications as described above.
DiagnosisTop
Aortic aneurysms are usually detected incidentally on imaging studies performed for unrelated indications.
1. Chest radiography (Figure 3.19-4, panels A and B) reveals dilation of the aorta (a normal aortic silhouette does not exclude an aneurysm of the ascending aorta).
2. Transthoracic echocardiography (TTE) (Figure 3.19-4, panel C) is a useful screening test of the ascending aorta; visualization of the aortic arch and descending aorta is more difficult. Transesophageal echocardiography (TEE) allows for assessment of the entire thoracic aorta except for the short distal segment of the ascending aorta.
3. Ultrasonography is a key technique for diagnosing abdominal aortic aneurysms.
4. Angiography: Computed tomography angiography (CTA) (Figure 3.19-5) allows for accurate assessment of the size (to the nearest 2 mm) and extent of an aneurysm as well as the anatomical relationships between the aneurysm, adjacent organs, and arteries branching off from the aorta (sometimes this is sufficient for the preoperative assessment of the patient). It is also used to detect coexisting aortic dissection, aortic intramural hematoma, and penetrating aortic ulcer. Magnetic resonance angiography (MRA) is useful for assessment of the size and extent of an aneurysm when CTA cannot be performed. It is particularly justified in serial periodic evaluations in younger patients. MRA is less useful in acute disease and does not reveal calcifications. Aortography with a calibrated catheter is sometimes used intraoperatively to confirm measurements prior to the final selection of an endovascular stent.
5. Intravascular ultrasonography allows for optimal visualization of the aortic wall during endovascular treatment.
In case of detecting an aortic aneurysm at any level of the aorta, consider imaging the remaining sections of the aorta and iliac arteries to exclude aneurysms in different locations and Doppler ultrasonography of the peripheral arteries to look for aneurysms. For aneurysms of the ascending aorta or aortic arch, examine the aortic valve (usually by echocardiography).
TreatmentTop
1. Management of cardiovascular risk factors, particularly smoking cessation and normalization of blood pressure (at least <140/90 mm Hg in patients without aortic dissection [see Essential Hypertension]).
2. Diagnosis and treatment of ischemic heart disease before elective invasive treatment of aneurysm.
3. Beta-blockers: Long-term treatment with oral beta-blockers slows down expansion of abdominal aortic aneurysms >40 mm in diameter but has no effect on the frequency of ruptures. Beta-blockers are also recommended in patients with thoracic aortic aneurysms and Marfan syndrome.
4. Losartan slows down expansion of the aortic root in patients with Marfan syndrome.
5. Surgical treatment usually involves implantation of a vascular prosthetic graft in place of an aneurysm. For asymptomatic aortic aneurysms, indications for repair depend on a number of factors including location, size, rate of growth, the presence or absence of aortopathy (Marfan syndrome, Loeys-Dietz syndrome), and overall patient condition including life expectancy. Those indications may include, in acceptable-risk patients with a life expectancy ≥2 years, >55 mm in diameter for the ascending aorta and aortic arch or >60 mm in diameter for the descending aorta. If possible, endovascular treatment is preferred. Other indications include smaller aneurysms in patients with Marfan syndrome and in those with a bicuspid aortic valve and risk factors; asymptomatic abdominal aortic aneurysms >55 mm in diameter in men (or >50 mm in women) or smaller if involving the suprarenal aorta, as the risk of repair is high in those cases; rapidly expanding aneurysms (in absolute terms ≥5 mm in 6 months or ≥7 mm in a year; more worrisome with a large relative size increase); and all symptomatic or ruptured aneurysms. Lower threshold values for invasive treatment may be used in patients with small body weight, with rapidly expanding aneurysms, with aortic regurgitation, those planning to become pregnant, or those with a borderline aorta diameter who prefer earlier invasive treatment. The recommended follow-up after surgery is duplex ultrasonography or computed tomography (CT) performed every 5 years.
6. Endovascular stenting has become the preferred treatment in most centers for patients with favorable anatomy, in particular of the descending thoracic aorta and infrarenal abdominal aorta (for patients at both low and high risk for open surgical repair). Patients with more complicated anatomy, such as with aneurysm involvement of the aortic arch, visceral vessels, or both, may be offered traditional surgery (if low risk) or custom-designed “fenestrated” or “branched” endovascular grafts if they are felt to be at higher risk of perioperative complications. Indications for endovascular repair (size, expansion, symptoms) are similar to those for open surgical treatment. The decision to proceed with endovascular repair over surgical repair is made after a discussion with the patient about considerations such as recovery time, durability of repair, feasibility, and risk, as noted earlier. Endovascular stents are increasingly being used in urgent and emergent circumstances in addition to their longstanding use in elective (planned) repairs.
Follow-UpTop
1. Screening for abdominal aortic aneurysms: Recommendations for screening may vary (from country to country) and are influenced by sex, family history, and history of tobacco use. Perform abdominal ultrasonography in all male patients >65 years (this may be also considered in female patients aged >65 years with a history of tobacco use) and consider it in >65-year-old first-degree relatives of patients with an abdominal aortic aneurysm. In patients with an aortic diameter of 26 to 29 mm, a subsequent imaging study is recommended after 10 years.
2. Asymptomatic thoracic aortic aneurysms: Perform CTA or MRA 6 months after detecting an aneurysm. Subsequent studies, using the same method at the same facility, are performed once every 12 months if the aneurysm is not expanding or every 6 months if the aneurysm expands significantly on subsequent studies.
3. Abdominal aortic aneurysms: Investigations may depend on the speed of development and expansion. Suggestions include follow-up ultrasonography or CT:
1) In patients with aneurysms 30 to 39 mm in diameter, every 3 years.
2) In patients with aneurysms 40 to 49 mm in diameter, every year.
3) In patients with aneurysms 50 to 54 mm in diameter, every 6 months.
4. In patients after surgery, our pattern of practice is to perform follow-up CT every 5 years and duplex ultrasonography at a year and then, depending on residual ectasia of the aorta, every year or 2 years.
5. In patients after endovascular surgery, imaging and regular surveillance are required to ensure that the stent graft remains in good position and there are no endoleaks (persisting blood flow in the aneurysm sac) that result in elevated pressure in the aneurysm sac and its expansion. For abdominal endovascular grafts, CT is performed at 1 month following implantation to assess graft position and check for endoleaks. Subsequent imaging with duplex ultrasonography or CT is performed for surveillance, with the choice of modality and interval for imaging depending on the results or stability of the repair and surgeon preference. Thoracic endovascular grafts cannot be adequately visualized with duplex ultrasonography and generally require CTA for follow-up, with the interval also determined by the results and surgeon preference.
ComplicationsTop
1. Aneurysm rupture manifests as a severe constant pain in the chest or abdomen with rapidly developing hypovolemic shock. Thoracic aortic aneurysms may rupture into the pleural cavity (usually on the left), mediastinum, pericardium (leading to rapidly developing cardiac tamponade), or esophagus (rare; this causes life-threatening hematemesis). Abdominal aortic aneurysms may rupture into the retroperitoneal space (producing a characteristic group of symptoms: sudden severe pain in the abdomen and lumbosacral region, hypovolemic shock, perineal and scrotal hematoma); peritoneal cavity (abdominal pain, symptoms of shock, and abdominal distention); duodenum (rare; massive gastrointestinal bleeding); inferior vena cava or renal or iliac vein (rare; symptoms of rapidly progressing heart failure with increased cardiac output).
2. Aortic dissection: The existing aneurysm rarely dissects; it is usually the dissection that may lead to expansion and aneurysm formation.
Tables and FiguresTop
|
Aortic segment |
Diameter (mm) |
Imaging study |
|
Aortic annulus |
F: 23 ± 2 M: 26 ± 3 |
Transthoracic US |
|
Sinus of Valsalva |
F: 30 ± 3 M: 34 ± 3 |
Transthoracic US |
|
Aortic root |
<37 |
Transthoracic US |
|
Proximal ascending aorta |
F: 26 ± 3 M: 29 ± 3 |
Transesophageal US |
|
Ascending aorta |
14-21/m2 BSA |
Transesophageal US |
|
25-38 |
CT | |
|
Descending aorta |
10-16/m2 BSA |
Transesophageal US |
|
17-28 |
CT | |
|
Abdominal aorta |
14-21 |
B-mode US |
|
BSA, body surface area; CT, computed tomography; F, female patients; M, male patients; US, ultrasonography. | ||
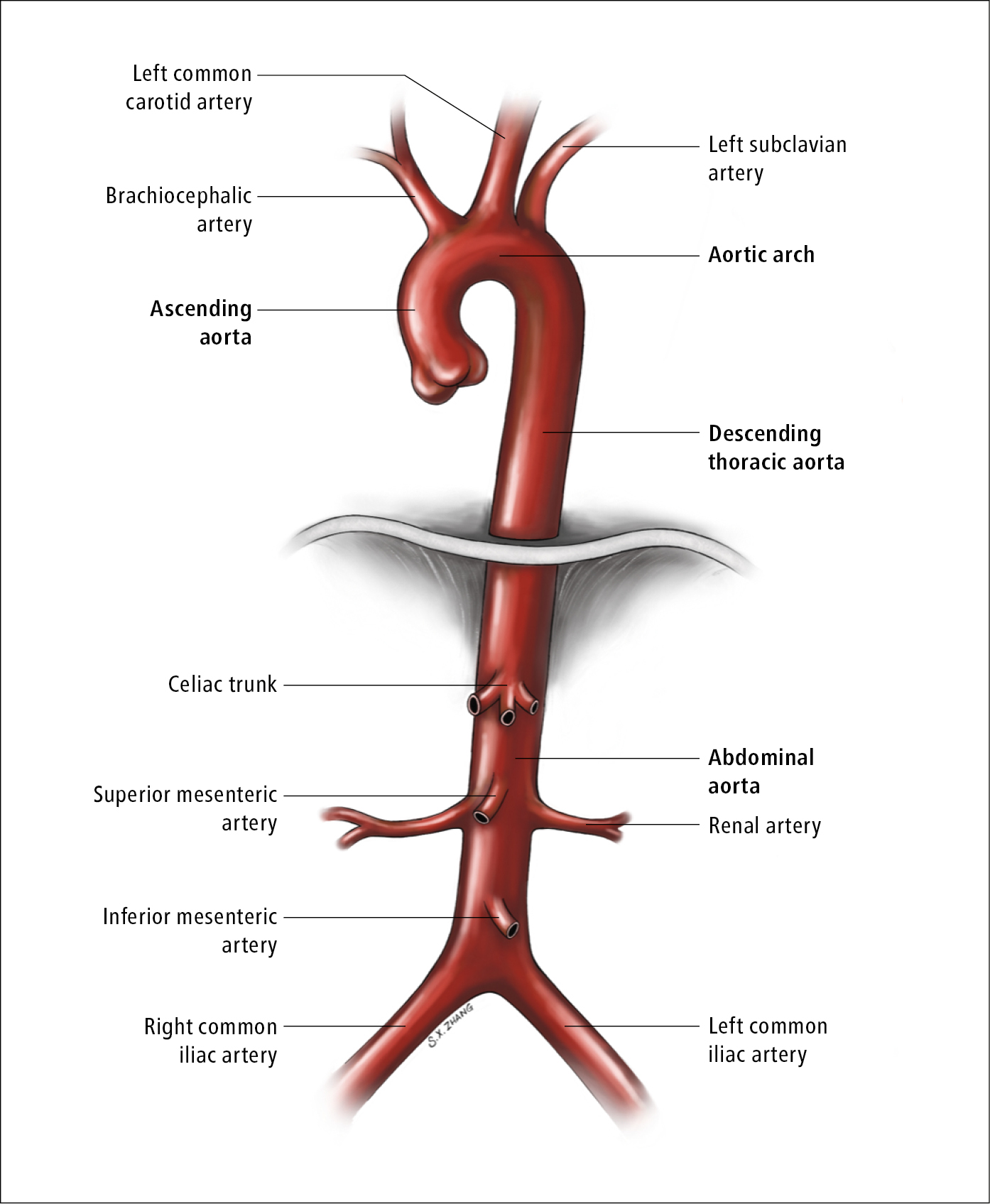
Figure 3.19-1. Anatomy of the aorta. Illustration courtesy of Dr Shannon Zhang.
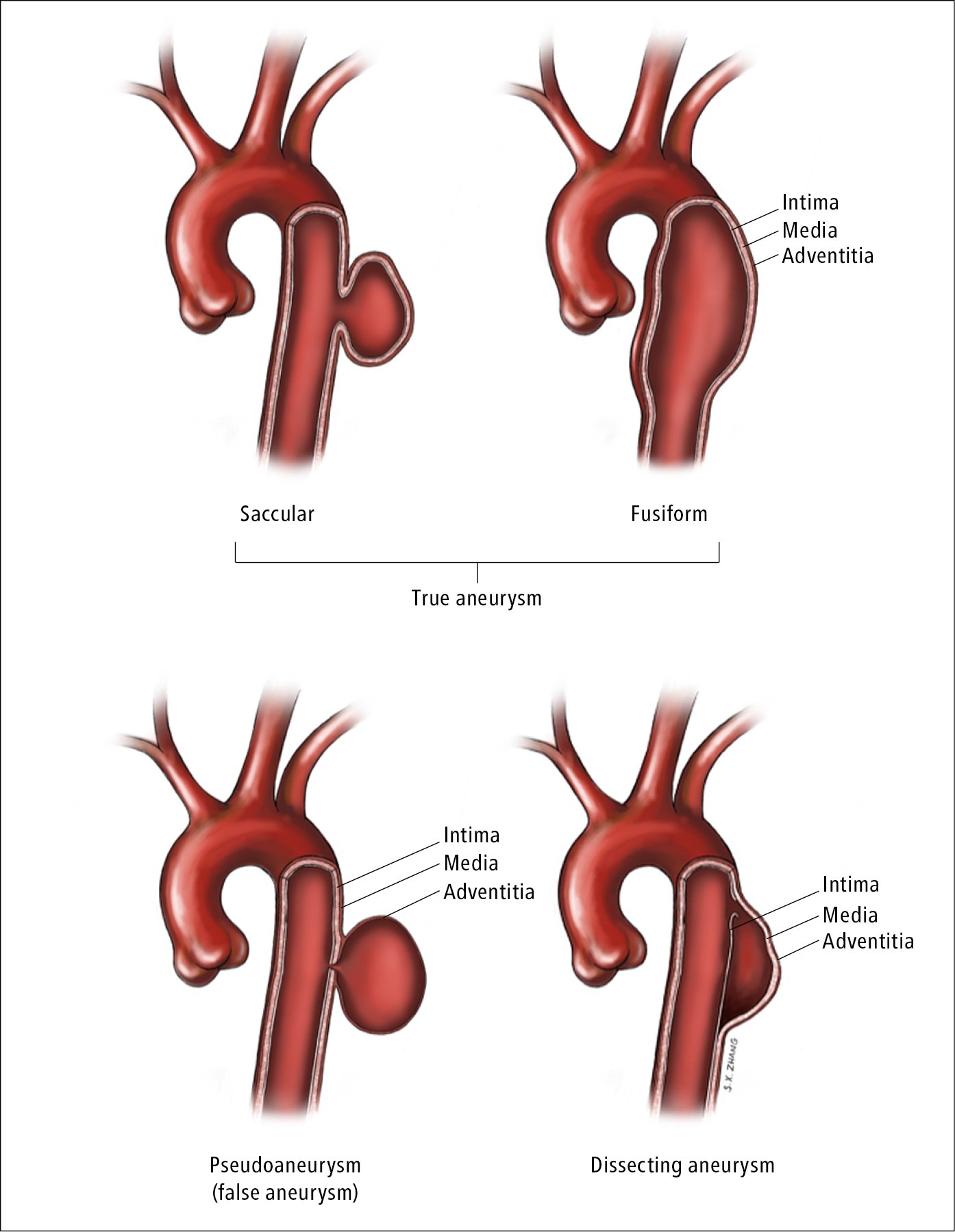
Figure 3.19-2. Different types of aortic aneurysm. Illustration courtesy of Dr Shannon Zhang.
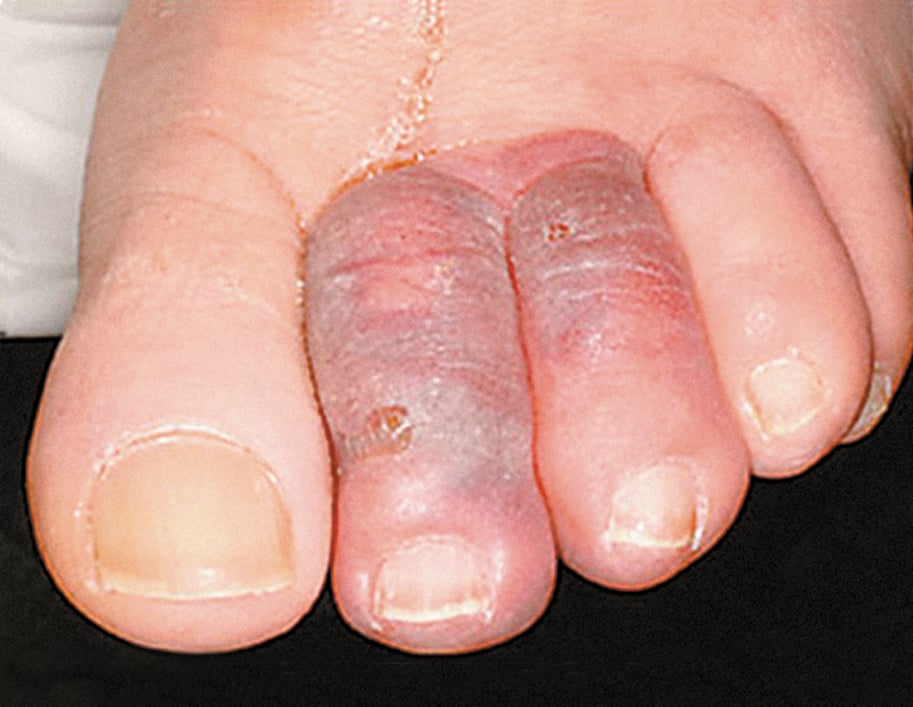
Figure 3.19-3. Arterioarterial embolism: blue toe syndrome (BTS). Figure courtesy of Dr Leszek Masłowski.
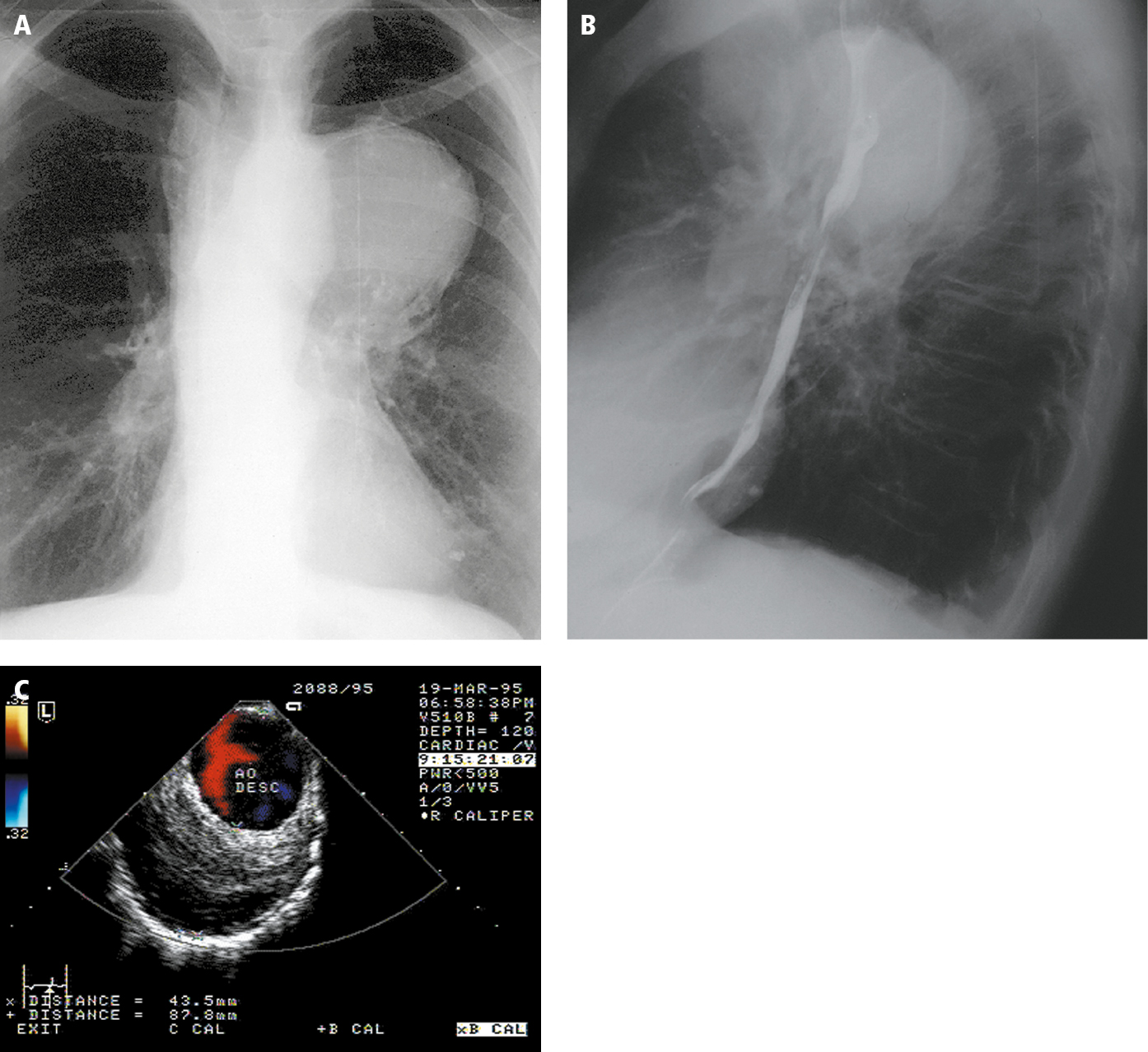
Figure 3.19-4. Descending aortic aneurysm. Chest radiography: posteroanterior (PA) view (A) and left lateral view (B). C, transesophageal color Doppler ultrasonography. Figure courtesy of Dr Marek Krzanowski.
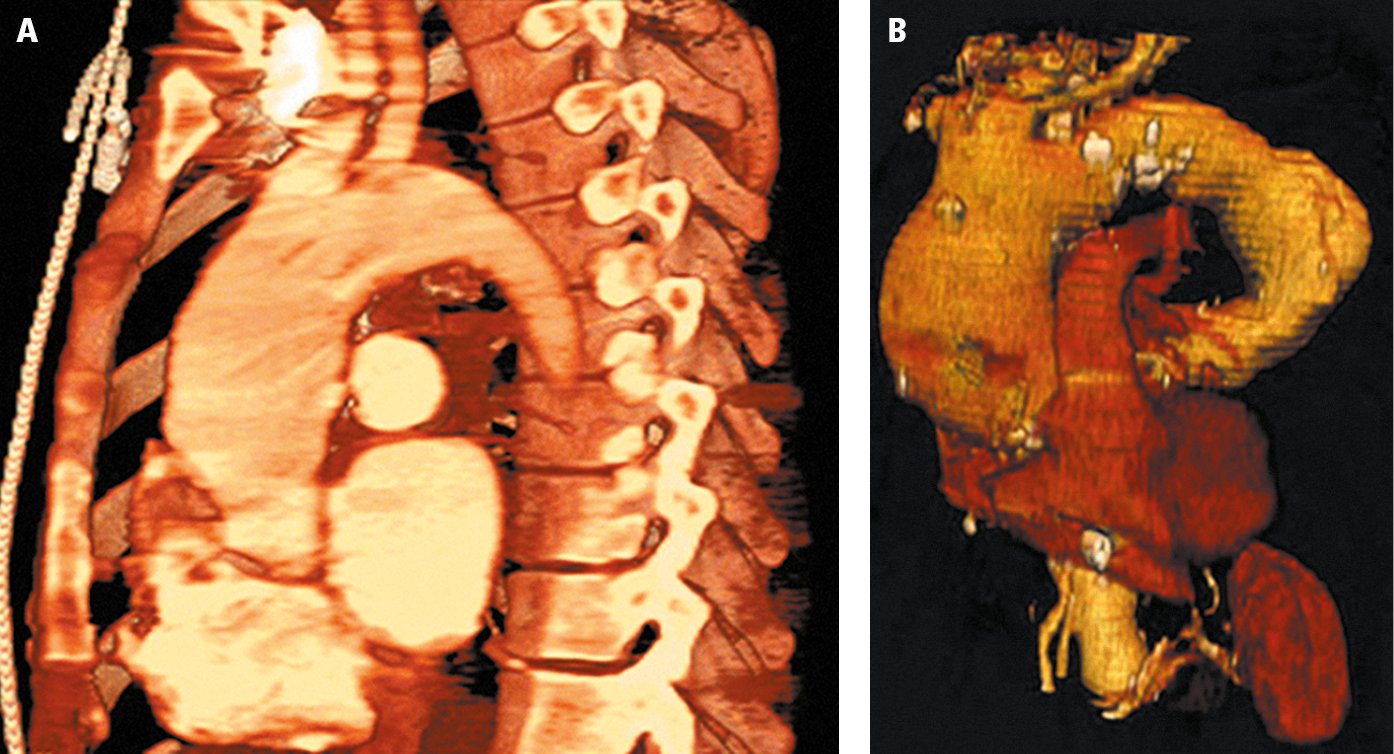
Figure 3.19-5. A, computed tomography (CT) of a thoracic aortic aneurysm; B, 3D reconstruction. Figure courtesy of Dr Leszek Masłowski.