Coisne A, Lancellotti P, Habib G, et al. ACC/AHA and ESC/EACTS Guidelines for the Management of Valvular Heart Diseases: JACC Guideline Comparison. J Am Coll Cardiol. 2023;82(8):721-734. doi:10.1016/j.jacc.2023.05.061
Vahanian A, Beyersdorf F, Praz F, et al; ESC/EACTS Scientific Document Group. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur J Cardiothorac Surg. 2021 Oct 22;60(4):727-800. doi: 10.1093/ejcts/ezab389. PMID: 34453161.
Baumgartner H, De Backer J, Babu-Narayan SV, et al; ESC Scientific Document Group. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur Heart J. 2021 Feb 11;42(6):563-645. doi: 10.1093/eurheartj/ehaa554. PMID: 32860028.
Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021 Feb 2;143(5):e35-e71. doi: 10.1161/CIR.0000000000000932. Epub 2020 Dec 17. Erratum in: Circulation. 2021 Feb 2;143(5):e228. Erratum in: Circulation. 2021 Mar 9;143(10):e784. PMID: 33332149.
Definition, Etiology, PathogenesisTop
Aortic regurgitation (AR) is a reversal of blood flow from the aorta into the left ventricle (LV) due to incomplete closure of the aortic valve leaflets. Primary regurgitation is caused by damage to or a congenital abnormality of the leaflets, with subsequent dilation of the left ventricular outflow tract, aortic annulus, and ascending aorta. Secondary regurgitation is caused by dilation of the aortic annulus and the ascending aorta (secondarily causing malcoaptation of the aortic valve leaflets) in the absence of significant aortic valve leaflet pathology.
Etiology:
1) Primary: Congenital (bicuspid aortic valve, quadricuspid aortic valve, valve damage in subaortic stenosis); degenerative (calcifications, fibrosis); infective endocarditis (active or healed); rheumatic; drug-induced (fenfluramine, phentermine) damage of the leaflets.
2) Secondary: Idiopathic aortic dilation; hypertensive aortic dilation; systemic connective tissue diseases (rheumatic disease, rheumatoid arthritis, aortic stenosis); dilation or dissection of the ascending aorta (hypertension, Marfan or Marfan-like syndrome, atherosclerosis, inflammation, trauma, myxomatous degeneration); aortopathy associated with bicuspid aortic valve; syphilitic aortic disease.
Clinical Features and Natural HistoryTop
1. Symptoms: In acute AR, a sudden-onset tachycardia and increasing dyspnea (in AR caused by aortic dissection, symptoms of the underlying condition predominate). Chronic AR may be asymptomatic for years, and even in severe AR, the symptoms are at times mild, often involving fatigue.
2. Signs: A wide (high) pulse pressure (with elevated systolic blood pressure and low, at times undetectable diastolic blood pressure), rapidly rising and rapidly collapsing pulse (so-called water hammer pulse or Corrigan pulse); sometimes a bisferiens pulse (more easily recognized on the brachial or femoral arteries than on the carotid arteries). The first heart sound is usually normal (although it may be silent in acute AR due to mitral valve preclosure). The aortic component of the second heart sound may be accentuated (in the case of aortic pathology) or soft (in the case of pathology of the leaflets). A holodiastolic decrescendo murmur is audible, frequently most prominent at the left sternal border (in the case of pathology of the ascending aorta, it is frequently better audible at the right sternal border); the Austin Flint murmur—a diastolic rumble due to relative mitral stenosis from preclosure of the mitral valve—may also be present. Frequently, a systolic ejection murmur is audible over the aortic valve (due to increased stroke volume, resulting in increased transaortic valve gradients).
3. Natural history of acute AR depends on the underlying condition. Chronic AR is usually asymptomatic for several years; in patients with a normal left ventricular ejection fraction (LVEF), the sudden cardiac death risk is <0.2% per year. The prevalence of cardiovascular events is ~5% per year in patients with severe AR and preserved LV function, and 25% per year in patients with New York Heart Association (NYHA) class III/IV symptoms. Some patients with asymptomatic severe AR may develop irreversible LV dysfunction; thus, early detection and appropriate treatment of severe AR is paramount.
DiagnosisTop
Diagnosis is based on typical clinical features and echocardiography.
1. Electrocardiography (ECG): Features of LV hypertrophy and strain pattern; features of left atrial enlargement (P mitrale). Ventricular arrhythmias can occur.
2. Chest radiography: LV hypertrophy, dilation of the ascending aorta and the aortic arch. In acute AR, pulmonary congestion with a normal cardiac silhouette is observed.
3. Doppler echocardiography (Figure 3.18-1) allows for detection of a regurgitation jet and its quantitative and qualitative assessment (Table 3.18-1).
4. Gated computed tomography (CT) is best for aortic dilation measurement using the inner-edge-to-inner-edge technique.
Echocardiography combined with clinical manifestations has very high accuracy in the diagnosis of AR. Consideration of the differential diagnosis of the causes of AR (primary valve damage and/or secondary to dilation of the aortic root or ascending aorta) is necessary to guide therapy.
TreatmentTop
1. Mild or moderate chronic AR: Asymptomatic patients with normal systolic LV function require no treatment aside from blood pressure control.
2. Chronic severe AR: In general, medical or surgical management may be considered with surgical opinion for potential aortic valve replacement (AVR) recommended in the presence of any of the following:
1) Symptoms attributable to AR (dyspnea, chest pain, orthopnea, paroxysmal nocturnal dyspnea).
2) No symptoms and severe AR with normal LV systolic function (LVEF ≥50%) but with severe LV dilation (LV end-systolic diameter >50 mm or 25 mm/m2 for patients with small body habitus).
3) LV systolic dysfunction (LVEF <50%-55%).
4) Progressive LV dilation (LV end-diastolic diameter >65 mm).
3. Acute symptomatic AR: Typically due to endocarditis or aortic dissection. Urgent surgery is required: AVR with the implantation of a mechanical or bioprosthetic valve, or an aortic homograft (details of invasive treatment: see below). Prior to surgery, vasodilators can be used. Aortic balloon counterpulsation is contraindicated.
AVR (mechanical vs bioprosthetic valve, depending on the patient’s age and ability to take warfarin [the latter necessary for mechanical prosthesis]) is the mainstay of surgical therapy in chronic severe AR and in acute symptomatic AR. In cases of AR caused by aortic root dilation where the aortic valve is structurally normal, surgical aortic root replacement may be performed with preservation of the native aortic valve leaflets. In cases of both aortic root enlargement and abnormal aortic valves, concomitant AVR and aortic root replacement (Bentall procedure) may be performed. Transcatheter valve replacement may be considered by experienced centers in patients who are ineligible for surgical AVR and in selected cases of AR related to bioprosthetic valve degeneration.
1. Vasodilators (agents: see Table 3.9-4), for instance, enalapril 10 to 20 mg bid, quinapril 10 to 20 mg/d, or losartan 50 to 100 mg bid (but also other angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers)Evidence 1Strong recommendation (downsides clearly outweigh benefits; right action for all or almost all patients). Moderate Quality of Evidence (moderate confidence that we know true effects of intervention). Quality of Evidence lowered due to the observational nature of the study but increased due to the relatively strong effect size. Elder DH, Wei L, Szwejkowski BR, et al. The impact of renin-angiotensin-aldosterone system blockade on heart failure outcomes and mortality in patients identified to have aortic regurgitation: a large population cohort study. J Am Coll Cardiol. 2011 Nov 8;58(20):2084-91. doi: 10.1016/j.jacc.2011.07.043. PubMed PMID: 22051330. may be used in:
1) Patients with hypertension.
2) Patients with severe AR who have symptoms and/or LV dysfunction when surgery is not performed because of comorbidities.
2. Prevention of infective endocarditis: see Infective Endocarditis.
Follow-UpTop
Follow-up: Table 3.18-2.
PrognosisTop
In symptomatic patients receiving medical treatment, 5-year survival rates are 30% for NYHA class III/IV patients and 70% for NYHA class II patients.
Tables and FiguresTop
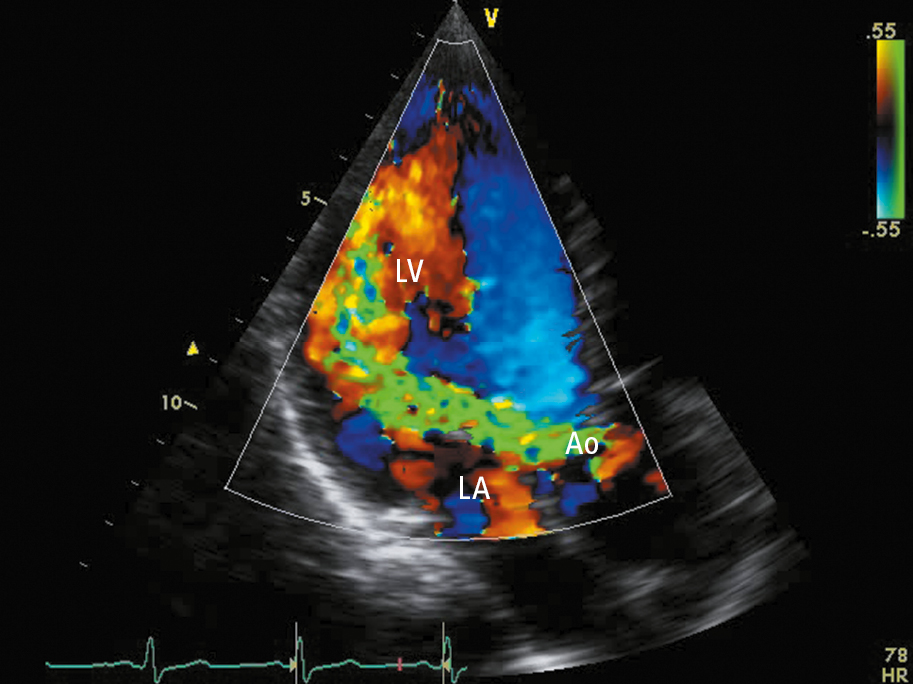
Figure 3.18-1. Transthoracic echocardiography (TTE) (apical long-axis view): severe aortic regurgitation (regurgitation jet marked in green). Ao, aorta; LA, left atrium; LV, left ventricle.
|
|
Regurgitation | ||
|
Mild |
Moderate |
Severe | |
|
Vena contracta width (mm) |
<3 |
4-6 |
≥7 |
|
Effective regurgitant orifice area (cm2) |
<0.10 |
0.1-0.29 |
≥0.30 |
|
Regurgitant volume (mL) |
<30 |
30-59 |
≥60 |
|
Regurgitant fraction (%) |
<30 |
30-49 |
≥50 |
|
Based on Circulation. 2014;129(23):e521-643. | |||
|
Severity of regurgitation |
Left ventricular function |
Follow-up |
|
Mild or moderate |
Normal LVEF and LVESD |
Every 2 years |
|
Severe |
LVEDD 60-65 mm |
Every yeara |
|
LVEDD >65 mm |
Every 6 months | |
|
Aortic dilation <50 mm |
Every yearb | |
|
a The first follow-up visit within 6 months of the diagnosis. b Increases >3 mm should be confirmed on CT. | ||
|
CT, computed tomography; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic diameter. | ||