Pereira J. The Pallium Palliative Pocketbook: a peer-reviewed, referenced resource. 2nd ed. Pallium Canada; 2016.
Twycross R, Wilcock A, Howard P, eds. Palliative Care Formulary. 5th ed. palliativedrugs.com Ltd; 2014.
Ripamonti CI, Santini D, Maranzano E, Berti M, Roila F; ESMO Guidelines Working Group. Management of cancer pain: ESMO Clinical Practice Guidelines. Ann Oncol. 2012 Oct;23 Suppl 7:vii139-54. PubMed PMID: 22997447.
National Collaborating Centre for Cancer (UK). Opioids in Palliative Care: Safe and Effective Prescribing of Strong Opioids for Pain in Palliative Care of Adults. Cardiff (UK): National Collaborating Centre for Cancer (UK); 2012 May. PubMed PMID: 23285502.
Caraceni A, Hanks G, Kaasa S, et al; European Palliative Care Research Collaborative (EPCRC); European Association for Palliative Care (EAPC). Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol. 2012 Feb;13(2):e58-68. doi: 10.1016/S1470-2045(12)70040-2. Review. PubMed PMID: 22300860.
The information provided in this chapter relates to palliating and managing pain caused by diseases such as cancer and advanced organ disease. While some of the approaches may also be appropriate for managing chronic pain caused by musculoskeletal or myofascial conditions, the reader is referred to guidelines specific to managing chronic pain, as some approaches differ.
DefinitionsTop
Pain is an unpleasant sensory and emotional experience that results from actual (eg, trauma) or potential (eg, tissue ischemia) tissue damage. Pain can be purely nociceptive, purely neuropathic, or mixed.
Nociceptive pain is caused by damage to soft tissues and to bone by disease or trauma. It is often divided into somatic pain and visceral pain. Somatic pain (eg, metastatic bone pain) is usually well localized. It is often described as a dull ache, gnawing pain, or sometimes as being sharp in quality. Visceral pain usually relates to pain caused by damage to viscera and organs. It can be colicky (eg, pain from bowel obstruction) or capsular, when organ capsules are stretched (eg, liver tumors). Visceral pain is less localized and can be referred along neural pathways (eg, shoulder pain from liver disease).
Neuropathic pain is associated with damage to the nervous system, such as with diabetic neuropathy, severed nerves during surgery, and compression or traction of a nerve by a tumor. Neuropathic pain often presents clinically as a burning sensation (dysesthetic type), shooting or lancinating pain (neuralgic type), or a combination of these sensations (mixed type).
Myofascial pain is either localized in or referred to a muscle. It is characterized by the presence of trigger points (an area of the muscle that fails to relax, forming a hard spindle-shaped nodule or band that is tender on palpation). Myofascial pain is both nociceptive and neuropathic in nature.
Breakthrough pain refers to a transient exacerbation of pain that occurs despite adequate management of background pain. Breakthrough pain can be nociceptive or neuropathic in origin. It can appear suddenly (over minutes) or have a gradual onset over many minutes to hours. It can be predictable, when a certain action predictably increases pain intensity, or unpredictable, when there is no pattern to its onset. Breakthrough pain that is associated with a particular activity (eg, walking with a large metastatic lesion in the hip, coughing in the case of multiple rib metastases) is referred to as incident pain.
Tolerance describes the reduced effectiveness of an opioid that occurs over time in many patients with advanced progressive disease. Tolerance develops due to disease progression and poorly understood changes that occur at the level of cellular receptors. Tolerance is different from addiction, which refers to psychological dependence that has individuals use a substance despite the harm that it causes. Addiction is frequently part of substance use disorder; tolerance is distinct from addiction or substance use disorder.
Withdrawal is the phenomenon where patients on long-term opioid therapy have symptoms related to abrupt cessation or significant reduction in their routine opioid dose. Symptoms can include anxiety, agitation, abdominal cramps and diarrhea, goosebumps, muscle aches, headaches, and watery discharge from the nose and eyes. Withdrawal occurs at a cellular level, as opioid receptors are suddenly unfilled. Withdrawal can also occur in addiction, but the presence of withdrawal does not imply addiction.
CausesTop
In patients with cancer causes of pain include:
1) Infiltration or compression of various tissues or organs by a tumor.
2) Complications of cancer (eg, pathologic vertebral fractures due to skeletal metastases).
3) Cancer treatment (eg, radiation-induced plexopathy, postmastectomy pain syndrome, chemotherapy-induced neuropathy).
4) Non–cancer-related pain or pain from comorbid conditions (eg, primary headache, angina).
In patients with pain related to advanced organ diseases causes and mechanisms of pain vary and can include:
1) Ischemic pain from poor peripheral perfusion (eg, congestive heart failure).
2) Neuropathic pain in neurologic illnesses (eg, dysesthesia from diabetic neuropathy or shooting pain from multiple sclerosis).
3) Pain that may occur from disease complications and progression (eg, calciphylaxis in end-stage kidney disease).
4) Pain that may occur independently from advanced disease (eg, headache or toothache) or as a result of comorbid conditions.
A new or dramatic worsening of pain should not be assumed to be the result of underlying cancer or advanced disease. It should be evaluated, within the scope of the patient’s goals of care, in order to identify its cause and best approach to therapy.
PharmacotherapyTop
Note that the recommended starting and maximal opioid doses vary across different sources, differing by 10% to 25% or more in some cases. Local regulations, patterns of practice, clinical context, and experience with these agents should always be taken into consideration.
1. Use oral analgesics. Whenever possible, oral medications should be used, as this route of administration is the least invasive. If oral treatment is not possible (due to nausea, vomiting, dysphagia), then subcutaneous, IV, or in selected cases transdermal routes are available.
Transdermal absorption can be unpredictable in patients with edema or reduced peripheral blood flow (including those actively dying). Initiation of transdermal medications should be avoided in such situations. However, if patients are already receiving these medications at the end of life and find them effective, there is no need to stop this therapy.
Subcutaneous injections, when appropriate for selected medication, are preferred over IV injections for patients at home and for those using long-term parenteral analgesics. Repeated IM injections are painful and offer no benefits over the subcutaneous or IV route.
2. Use analgesics in accordance with the World Health Organization (WHO) analgesic ladder (Figure 1.32-1). The WHO analgesic ladder guides therapeutic decisions based on pain intensity.
In patients with mild pain (rated at 1-3 on a 10-point scale with minimal impact on function and mood), start at step 1 using acetaminophen (INN paracetamol), nonsteroidal anti-inflammatory drugs (NSAIDs), and other nonopioid medications, unless contraindicated.
Step 2 is for patients with moderate pain (rated at 4-6/10). A weak opioid such as codeine or tramadol is used as the main analgesic at this step. The medication may be either added to those used in step 1 if there is merit in their continued use or it may replace the previous medications.
Patients with severe pain (rated at ≥7/10 and with significant impact on functioning and mood) generally need a strong opioid (eg, morphine, hydromorphone, oxycodone) regardless of the underlying mechanism of pain. Any step 2 medications currently being used should be discontinued or reassessed at this time to avoid medication duplication or polypharmacy.
At any step of the analgesic ladder, if therapeutic results are unsatisfactory, clinicians are advised to progress to the next step of the ladder in attempt to achieve better pain control and increased function. There is no reason to start at step 1 or even step 2 if pain is severe.
At each of the steps an adjuvant analgesic can and should be used, depending on the type of pain. A tricyclic antidepressant, gabapentin, or pregabalin could be considered for neuropathic pain, and an anti-inflammatory agent could be considered for bone pain. These coanalgesics and nonopioid medications improve analgesia, reduce opioid requirements, and inhibit the development of opioid tolerance.
3. In patients with ongoing pain, analgesics should be administered on a regular basis. Regular (around-the-clock) administration of analgesia provides more consistent pain control than intermittent, as-needed (pro re nata [prn]) dosing. The dose interval should be adjusted to the pharmacokinetics of the specific medication.
4. Patients with ongoing pain should have immediate-release analgesics available on an as-needed basis for breakthrough pain. These as-needed (prn) doses are intended to treat predictable and unpredictable exacerbations of pain.
For patients on a routine (around-the-clock) opioid regimen, their prn schedule is typically the same opioid at 1/10 to 1/6 of their total daily dose usually every 2 to 4 hours.
The prn analgesic may be a different opioid if there is no short-acting formulation of the patient’s around-the-clock opioid. For example, a fentanyl patch for routine pain control would require another type of strong opioid, such as morphine or hydromorphone, in a short-acting formulation for prn dosing by the oral route; in some locations mucosal forms of fentanyl are available for intranasal or sublingual use and could be used as the prn medication of choice.
A small proportion of patients with advanced cancer or other advanced illnesses may only have incident pain sporadically during the day, associated with specific activities or times of day, rather than constant or frequent pain episodes. These patients may only need a prn analgesic to be taken prior to these predictable episodes of incident pain.
In order to optimize comfort and function, changes in the baseline regimen should be considered when patients require more than 3 to 4 prn doses per day.
Patients are encouraged to keep a pain diary to record the use and impact of the breakthrough doses (and regular doses) to aid pain assessment and dose adjustments.
5. Titrate the analgesic dose to effect. Increasing or decreasing the dose of the analgesic to ensure good pain control and avoid intolerable adverse effects is a mainstay of treatment, especially with opioids. After starting any analgesic regimen, the patient should be reassessed daily (in an inpatient setting) or every 3 to 4 days (in a community/home setting) to adjust the dose.
Titration of opioids should be guided by the total daily dose. Adding the total of all prn doses to the routine around-the-clock doses is a common way to titrate analgesia (eg, 6 mg prn in 24 h would be an extra 1 mg to each of the 4-hourly routine doses of the analgesic). In other situations, such as when patients are unable to ask for prn medications or their prn use seems disproportionate to the discomfort they are in, clinicians may choose to increase the routine doses by 20% to 50%, depending on the overall opioid dose range being used (lower increase for higher doses) and the level of ongoing pain.
Titration ends when pain is well controlled and the patient is using ≤3 breakthrough doses a day without developing distressing adverse effects.
Once pain is well controlled, the patient can be switched to a long-acting opioid formulation (of the same opioid type or another if no long-acting formulation is available) for convenience and better adherence.
Sometimes downtitration may also be needed, for instance, when the patient is experiencing excessive adverse effects or when they have responded very well to another treatment (eg, radiotherapy) that has reduced the need for analgesics.
To avoid severe withdrawal symptoms and a pain crisis, opioid treatment should not be abruptly stopped in a patient who has been on long-term opioid therapy (eg, >10-20 days).
When complete opioid discontinuation is needed in a patient who has been receiving long-term opioid therapy, undertake a gradual weaning over many days if possible, reducing the dose by ~20% to 30% every 3 days or so until the patient is off the opioid.
6. Controlled-release (long-acting) opioid formulations should be used only after good pain control and stable dosing is achieved with immediate-release formulations. Controlled-release opioids provide analgesia with longer intervals between doses, allowing for patient convenience. They cannot be titrated as fast as immediate-release formulations. Controlled-release formulations should not be used in opioid-naive patients.
Once pain is well controlled, the patient can be switched to a long-acting opioid formulation (of the same opioid type or another if no long-acting formulation is available) for convenience and better adherence.
7. Always use lower starting doses in the very frail or frail elderly (see Frailty) and those with advanced renal, pulmonary, cardiac, or neurologic dysfunction. Opioid metabolism is slower in the elderly, increasing the risk of opioid accumulation and adverse events such as somnolence, delirium, or an inadvertent overdose.
Many opioids and their metabolites are excreted, to varying degrees, by the kidneys. This requires lower doses in patients with renal impairment. Buprenorphine and fentanyl are notable exceptions that are not known to be renally excreted.
Lower starting doses and slower titration are also required in patients with advanced heart, lung, kidney, or liver disease. Note that opioids in patients with advanced heart, lung, and neurologic diseases are effective for pain and dyspnea and are safe provided they are initiated at low doses and titrated slowly.
1. Acetaminophen has a rapid onset (15-30 min) and short duration of action (up to 4-6 h). The maximum daily dose in adults (>12 years) who are not at increased risk of hepatotoxicity is 3 to 4 g/24 h. Doses ≤2.6 g/d are recommended in the elderly and even lower doses are recommended in those with liver disease or with regular alcohol use.
2. NSAIDs may be useful for patients with mild pain or as adjuvants for patients with metastatic bone pain. The onset and duration of action varies by formulation. The agents include ibuprofen, with a rapid onset (15-30 min) and short duration (4-6 h) of action, and celecoxib, with a slower onset (3 h) and long duration (14 h).
Before using NSAIDs, the risk of cardiovascular, gastrointestinal (GI), and renal adverse effects must be evaluated. The use of selective cyclooxygenase-2 (COX-2) inhibitors, proton pump inhibitors (PPIs), or both will reduce—but not eliminate—the risks of GI bleeding. There is no evidence that COX-2 inhibitors have a reduced renal or cardiac risk compared with nonselective COX inhibitors.
Patients who are frail and have advanced disease may not tolerate NSAIDs very well.
The provided trade (brand) names are valid for Canada.
Weak opioids are usually used for moderate pain. Unlike strong opioids, they have ceiling doses that when exceeded do not necessarily improve pain but increase the risk for adverse effects. They are often available in combinations with nonopioids such as acetaminophen, which can further limit the ceiling dose.
1. Codeine is a prodrug that exerts its analgesic effects once metabolized to morphine (mainly) and hydrocodone (to a lesser degree). Patients with poor or rapid CYP2D6 metabolism do not respond well to codeine.
Suggested starting doses: 15 mg every 4 hours (see Table 1.32-1). If necessary, titrate the dose to a maximum of 240 to 300 mg/d.
Immediate-release and controlled-release formulations are available. Immediate-release formulations contain codeine alone or codeine combined with acetaminophen (marketed as Tylenol with codeine) or with acetylsalicylic acid (ASA) (previously available as 222), limiting the maximum daily dose. Oral formulations are the only formulations available in Canada.
Codeine should not be used in severe renal impairment.
2. Dihydrocodeine (not available in Canada) is a synthetic opioid. Immediate-release formulations are available as dihydrocodeine alone or combined with acetaminophen, ASA, ibuprofen, antihistamines, or decongestants, limiting the maximum daily dose.
The starting dose is 60 mg orally every 12 hours. If necessary, titrate the dose up to 120 mg every 12 hours.
3. Tramadol is a weak opioid with a dual mechanism of action. It is an opioid receptor agonist and inhibitor of the reuptake of norepinephrine and serotonin. Like codeine, tramadol requires metabolism to an active metabolite, O-desmethyltramadol (M1), to be fully effective. Both tramadol and its M1 metabolite exert analgesic effects.
Suggested starting doses: From 25 mg to 37.5 mg to 50 mg every 8 h (see Table 1.32-1). Tramadol is usually initiated with an immediate-release oral formulation. Titrate the dose as needed at a rate no faster than 25 mg every 3 days up to a maximum of 400 mg/d.
Immediate-release and controlled-release formulations are available. Immediate-release formulations contain tramadol alone or tramadol combined with acetaminophen (marketed as Tramacet), limiting the maximum daily dose. Oral formulations are the only formulations available in Canada.
Do not use tramadol in patients with poorly controlled seizure disorders. Tramadol is associated with serotonin syndrome, although this is uncommon.
Strong opioids generally do not have a set ceiling dose. The maximum dose is determined clinically on an individual basis and is reached when intolerable adverse effects start occurring or there is no response despite adequate titration (indicating poor response or tolerance to that opioid by the specific individual). There is considerable interindividual and intraindividual variability in response to opioids regarding both the analgesic and the adverse effects. Different responses to the same opioid dose are seen in different individuals with similar disease profiles. Also, the same individual can have different responses to different opioids.
When prescribing, avoid giving a dose range, as ranges may result in variability in administration (sometimes one dose and other times another in the range). Prescribe one dose, monitor its impact, and then increase the dose if more analgesia is needed.
Also see Opioids and Opioid Use Disorder: General Considerations.
1. Morphine is still considered internationally as the reference strong opioid. While there are regional differences in practice, it remains a useful first-line strong opioid for starting opioid treatment. Allergy to morphine is rare and if it is confirmed of suspected, morphine should be avoided. Nausea or somnolence alone is not considered an allergy, although many patients report allergy in the presence of symptoms; with education and management of adverse effects, morphine can be used in such cases.
Suggested starting doses: 5 mg orally every 4 hours (see Table 1.32-1). Titrate the dose to achieve adequate analgesia with no or minimal adverse effects. Titration can be done every 1 to 2 days depending on the clinical response (analgesia and adverse effects) and may require a dose increase of ≥25% to 50%.
Immediate-release and controlled-release formulations are available. Oral, rectal, and parenteral (for subcutaneous or IV administration) formulations are available in Canada. Conversion between different routes of administration: Table 1.32-2, Table 1.32-3.
Morphine and its active metabolites are excreted by the kidneys. Morphine can be used in patients with mild to moderate renal impairment but at lower starting doses and with slower titration. Morphine is contraindicated in patients with severe renal impairment and renal failure.
2. Hydromorphone is a semisynthetic morphine-based opioid. Like morphine, it has active metabolites that are excreted renally. It can be used at lower doses in patients with mild to moderate renal impairment but should be avoided in patients with severe renal impairment or renal failure. Hydromorphone is not superior to morphine in terms of its analgesic and adverse effect profile. Patients with a true allergy to morphine should avoid hydromorphone, as there can be cross-reactivity for allergy.
Suggested starting doses: 1 mg every 4 hours (see Table 1.32-1; take frailty into account. Titrate the dose to achieve adequate analgesia or based on adverse effects. Immediate-release and controlled-release formulations are available. Oral and parenteral formulations, for subcutaneous and IV administration, are available in Canada. Conversion between different routes of administration: Table 1.32-2, Table 1.32-3.
3. Buprenorphine is a semisynthetic opioid available in Canada as a long-acting transdermal formulation. Buccal and sublingual formulations are available outside of Canada. Dosing is based on prior exposure to opioids. Buprenorphine can be used in opioid-naive patients at low doses with caution.
Transdermal patches (up to 20 microg/h) are changed every 7 days. Dose adjustments should occur only after 3 to 7 days. Higher-dose preparations of transdermal patches (from 35 microg/h) changed every 3 to 4 days are used in some European countries.
Buccal film and sublingual tablets are taken every 12 hours. Dose adjustments should occur no quicker than every 4 days. Lower doses and slower titration are advised in patients with oral lesions.
Sublingual tablets that contain both buprenorphine and naloxone are available in 2 dosage forms, but currently in Canada these are approved only for opioid maintenance therapy in opioid use disorder.
All regular dosing of alternative opioids should be discontinued when buprenorphine is started. Intermittent breakthrough opioids can be used to prevent increases in pain or symptoms of withdrawal. Buprenorphine is safe for use in patients with renal failure. Conversion from other opioids can be guided: Table 1.32-2.
4. Fentanyl is a strong opioid that, in the context of managing chronic pain related to advanced disease, is typically reserved for opioid-tolerant patients. It is available as controlled-release (transdermal patches) and immediate-release (transmucosal and IV/subcutaneous) formulations. Fentanyl is safe for use in patients with severe renal impairment and failure.
Parenteral formulations (for IV/subcutaneous dosing) should (1) be reserved for selected patients who need a rapid switch to fentanyl; (2) be used in monitored settings, such as operating rooms, intensive care units, and palliative care units; (3) involve continuous infusion (due to a very short half-life); and (4) be supervised by clinicians experienced in its use.
Breakthrough doses of fentanyl can be added to infusions, which are usually done using administration pumps, with or without patient self-administration of the breakthrough doses (with provisions for locking out for security and safety). In this context, fentanyl is ~100 times more potent than morphine (morphine 2-3 mg used orally is equivalent to 1 mg used subcutaneously or IV, which is equivalent to ~10 microg of fentanyl IV or subcutaneously).
Fentanyl buccal (transmucosal) or intranasal formulations are available in some jurisdictions for the management of severe breakthrough pain or breakthrough pain not responding to other short-acting opioids on an as-needed basis. There are some restrictions with these: patients must already be on ≥60 mg/d of oral morphine equivalent (OME), ≤4 breakthrough doses should be administered per day, and usually there should be an interval of ≥3 to 4 hours between each administration. Formulations are usually available at starting doses of 100 or 200 microg/d. Start with the lowest available dose of the formulation, monitor for effect, and titrate up to the next available dose if this is not effective after ≥3 consecutive trials.
Transdermal fentanyl is not recommended for patients using <60 mg/d of OME (some references suggest it can be used in patients stable on 45-60 mg of OME). Starting doses are determined using equianalgesic dose tables (Table 1.32-3) and are based on the dose of the opioid the patient is being switched from. Note that the tables display wide equianalgesic dose ranges, making conversion to a fentanyl patch less precise than to another opioid type. A switch to fentanyl patches should be considered once the patient’s pain is well controlled on a short-acting or long-acting opioid and the patient is using few (≤3) breakthrough analgesic doses in 24 hours without having intolerable adverse effects.
Controlled-release formulations such as the fentanyl patch should be titrated no sooner than every 3 to 4 days (preferably no sooner than once a week).
After application of a transdermal patch it takes 8 to 12 hours for the drug to reach effective analgesic blood levels. Thus, when initiating a fentanyl patch there should be an 8- to 12-hour overlap between patch application and discontinuation of the regular dose of the previous opioid. Similarly, when being discontinued, the patch should be removed and the new opioid should be started only 12 hours later to allow the drug to be fully eliminated. During that 8- to 12-hour interim period, breakthrough pain can be managed with breakthrough doses of analgesia.
For breakthrough pain, another short-acting opioid (eg, morphine or hydromorphone) can be used, prescribed only on an as-needed basis, at a dose of 10% or one-sixth of the total morphine equivalent dose the patient is on, as calculated using the conversion table of morphine to transdermal fentanyl: Table 1.32-3.
Occasionally patients on a fentanyl patch may experience a pain crisis, opioid neurotoxicity, or excessive somnolence and may require conversion from the transdermal patch (long-acting formulation) to parenteral fentanyl (short-acting formulation) to allow for more rapid dose uptitration or downtitration. In such situations consult a palliative care or pain specialist to assist with the switch.
Note that deaths have occurred in Canada when opioid-naive patients were started on fentanyl patches, even when applying the smallest dose of the patch.
5. Methadone, although often associated with opioid maintenance therapy for opioid use disorder, is a very useful opioid for the management of pain secondary to advanced diseases such as cancer. It is prescribed very differently for pain management compared with opioid maintenance therapy.
For the management of severe chronic pain, methadone may serve as the primary opioid (regular regimen) or it can be added to another opioid regimen as an adjuvant analgesic (a very small dose of 1 mg or 2 mg once a day or bid).
Methadone is an opioid with the onset of action of ~10 to 15 minutes and an intermediate duration of action requiring 8-hourly or 12-hourly oral administration. Only the oral formulation is available in Canada, but parenteral formulations are available in other jurisdictions.
Methadone has unique pharmacokinetic properties, including a long but unpredictable elimination half-life, high lipophilicity, and a variable equianalgesic dose ratio. This equianalgesic dose varies according to the dose of the opioid the patient is receiving prior to the switch to methadone (Table 1.32-2). If the patient is on <90 mg/d of OME, then methadone is ~4 times more potent; for ~300 mg/d of OME, methadone is ~8 times more potent; and for 500 mg/d of OME, it is ~10 times more potent. This unique feature of methadone, together with other distinct pharmacokinetic properties of this useful and effective opioid, requires that it only be prescribed and monitored by clinicians skilled in its use. Comfort and experience is especially critical during the time of switching to or from other opioids.
Methadone may be used in patients with moderate to severe renal impairment with a small reduction in dose. It is primarily metabolized in the liver by cytochrome P450 3A4 (CYP3A4); therefore, avoid medications that can inhibit this metabolism (eg, systemic antifungals, antiretrovirals, and some antibiotics, anticonvulsants, and antidepressants), which could lead to an unintentional opioid overdose.
Methadone may also prolong the QT interval. It needs to be used with caution in patients with cardiac dysrhythmias and those using medications that prolong the QT interval.
Obtain advice from a palliative care or pain specialist when considering methadone.
6. Oxycodone is a strong opioid with no fixed ceiling dose. The starting dose for oxycodone alone is 2.5 or 5 mg orally of the immediate-release formulation every 4 hours.
In Canada, oxycodone is available only in immediate-release and controlled-release oral formulations. Immediate-release formulations contain oxycodone alone or combined with acetaminophen (marketed as Percocet). Oxycodone is also available in combination with naloxone (marketed as Targin), which helps minimize the adverse effect of constipation.
The presence of codrugs in oxycodone formulations limits the maximum daily doses that are safe and recommended.
Management of Adverse Effects of Opioids
Also see Opioids and Opioid Use Disorder: General Considerations.
1. Sedation/somnolence usually occurs at the beginning of opioid treatment or after a considerable dose increase and resolves within a few days. If somnolence persists or worsens, the dose of the opioid can be decreased to the lowest dose that ensures pain control. Other causes of somnolence (eg, delirium, sedating medications, dehydration, brain metastases, hypercalcemia, infections, disease progression) should be excluded and treated where possible. Persistent somnolence may be an indication for switching to another opioid.
Patients should be advised not to drive or operate heavy equipment until their pain is well controlled, they are not requiring breakthrough doses, and they are experiencing no intolerable adverse effects, including somnolence. They should stop driving or operating equipment every time the dose is adjusted until they are stable again. Note that in some countries driving when using opioids is not allowed.
2. Nausea and vomiting may develop in the first days of starting an opioid or following a dose increase and usually resolve spontaneously. Opioids can elicit nausea and vomiting through direct effects on the chemoreceptor trigger zone in the brain (where dopamine is the main neurotransmitter) and the vomiting center in the brain as well as by way of reduced gut motility (with or without constipation). Patients should be informed of the risk of nausea and vomiting and made aware that these are likely to be transient (to prevent patients from discontinuing the drug on their own).
Patients should be prescribed an antiemetic medication for the first few days of opioid treatment (or when the opioid dose is increased). The most appropriate antiemetics are antidopaminergic agents that promote gut motility (eg, metoclopramide, domperidone). Ondansetron and haloperidol—antidopaminergic agents without the motility effect—can be effective but should be reserved for patients with severe opioid-induced nausea not responding to or unable to tolerate metoclopramide or domperidone.
Other causes of nausea and vomiting (eg, other medications, renal failure, disease progression, constipation, dehydration) should be excluded and treated where possible.
If nausea persists for more than a few days after opioid initiation or dose increase and does not respond to antiemetics, consider switching to a different type of opioid.
3. Constipation is the most frequent adverse effect of opioids. It generally does not resolve spontaneously and requires ongoing laxative treatment for as long as the patient is on an opioid. Patients using opioids on a regular basis should be therefore provided with prophylactic laxative therapy.
A number of laxatives are available, with different mechanisms of action. The most frequently used ones are stimulants (senna), osmotics (lactulose, polyethylene glycol), and combination agents (bisacodyl is both a stimulant and a contact laxative). Any of the laxatives may be used as first-line therapy. Doses should be titrated to ensure a bowel movement at least every 2 or 3 days. Some patients may require combinations of these agents. Docusate is ineffective.
Before laxatives are initiated, bowel obstruction should be excluded in patients with severe constipation and in those with constipation, vomiting, or abdominal pain.
4. Hallucinations, agitation, and cognitive dysfunction (presenting as delirium; see Delirium) may occur at the initiation of opioids, with dose increases, or more often after longer-term treatment by way of accumulation of active metabolites.
Distressing hallucinations and agitation may require temporary treatment with small doses of an antipsychotic (eg, haloperidol 0.5 or 1 mg orally or subcutaneously every 6-8 h as needed), including atypical antipsychotics (eg, quetiapine).
Subtle cognitive changes may occur in the absence of clinically evident delirium. These adverse effects are caused by active opioid metabolites, the opioid itself, or both, and can be reduced by decreasing the opioid dose to the lowest dose that provides adequate pain control. If severe, consider switching (rotating) to a different opioid.
Other causes of cognitive dysfunction or delirium (eg, other medications, renal failure, disease progression, or hypercalcemia) should be excluded and treated where possible.
5. Hyperalgesia and allodynia (sometimes referred to collectively as hyperalgesia) are uncommon and poorly understood phenomena where opioids lead to overstimulation of nociceptors and the resulting sensation of pain or oversensitivity to noxious and even normal stimuli, such as light touch. Hyperalgesia and allodynia, like hallucinations and agitation, are usually noted in the context of opioid neurotoxicity (see below). Patients with hyperalgesia/allodynia experience no or limited pain relief with increasing opioid doses. Opioid-induced hyperalgesia is a diagnosis of exclusion and alternative causes of pain, such as disease progression, must be excluded. For treatment approaches, see opioid neurotoxicity below.
6. Opioid-induced neurotoxicity is a clinical syndrome that presents as ≥1 of the following opioid adverse effects: hallucinations, agitation, somnolence, myoclonus, hyperalgesia, allodynia.
Opioid neurotoxicity does not appear to be mediated through opioid receptors and thus does not respond to opioid antagonists such as naloxone. If neurotoxicity is mild to moderate and pain is well controlled, the opioid dose can be reduced by 20% to 25%. If neurotoxicity is severe or pain is poorly controlled, an opioid rotation (switch from one type of opioid to another) should be considered. In all severities of neurotoxicity, artificial hydration (via subcutaneous or IV route) may be needed to enhance the elimination of active metabolites.
Specific symptoms can be managed with directed therapies, such as a benzodiazepine (to manage myoclonus) or an antipsychotic (to manage hallucinations). However, these approaches do not address the underlying cause of the toxicity and may aggravate cognitive dysfunction and delirium. They are reserved for situations when opioid switching is not possible (eg, the last few days of life).
In the final days of life, a switch from the oral to a parenteral route (eg, subcutaneous) may be considered for patients on morphine or hydromorphone (reduced first pass effect with some reduction in metabolite production).
7. Overdose can happen in those using opioid therapies at the end of life or in advanced disease, although it is rare. Overdose in this context is typically due to rapid dose increases, accidental dose increases (drug interactions, accidental ingestion), or underlying changes in physiology (eg, dehydration).
Note that management of opioid overdose in advanced disease and in the context of palliative care is different than management of opioid overdose in opioid use disorder, accidental ingestion, or acute pain management (eg, postoperatively). Overdose in this section is discussed only in the context of advanced disease and palliative care.
Features of opioid overdose in patients taking opioids in advanced disease include respiratory depression, miosis, and reduced level of consciousness.
Management of a mild opioid overdose (mild respiratory depression and mild reduction in the level of consciousness) includes withholding the next opioid dose and reducing the regular opioid dose (by ≥25%) in addition to IV or subcutaneous hydration. Low doses of naloxone (0.04 mg IV or subcutaneously) can be used if necessary but often this is not needed.
Management of a severe, life-threatening opioid overdose (respiratory depression, miosis, and unresponsiveness) requires immediate administration of an opioid antagonist (naloxone) at a full dose (see exceptions below). These doses may be repeated until the overdose is reversed. As the half-life of naloxone is shorter than that of therapeutic opioids, some patients require a continuous infusion of naloxone until the opioid is eliminated.
For patients with advanced disease who need pain management but develop an opioid overdose (which is rare), a balance is needed between reversing the overdose and maintaining analgesia (while avoiding withdrawal and a pain crisis); small aliquots of naloxone and/or infusions of smaller doses may be needed to achieve this.
Note that the dying process in patients with advanced disease involves gradually reduced respirations and reduced consciousness (see Last Days and Hours). In the absence of miosis do not administer naloxone, as this will result in unnecessary suffering at the end of life. If there is an opioid overdose (eg, severe miosis), withhold the opioid dose and reduce the subsequent doses instead of giving naloxone.
8. Other adverse effects: Dry mouth, pruritus, urinary retention, and sweating are less common opioid adverse effects that can be treated symptomatically. With long-term opioid use, suppression of luteinizing hormone (LH), follicle-stimulating hormone (FSH), adrenocorticotropic hormone (ACTH), and growth hormone (GH) secretion can occur, which can be associated with osteoporosis.
Opioid Rotation (Opioid Switching)
Opioid rotation (switching from one opioid type to another) is a useful strategy to manage opioid neurotoxicity, intolerable adverse effects of a particular opioid, poor response to an opioid despite titration, and lack of availability of a specific route or dose for a specific opioid. Opioid rotation leverages the considerable interindividual and intraindividual variability that exists in response to opioids (based on genetic variability in the expression of opioid receptors and metabolism).
Rotation involves switching from the current opioid (short-acting or long-acting formulation) to a short-acting formulation of another opioid. The short-acting formulation of the new opioid is chosen to allow for rapid titration up or down, which cannot be done with a long-acting formulation. If you are not acquainted with opioid rotation, contact a palliative care or pain clinician for advice on undertaking the switch.
Generally, an opioid switch is performed by (1) calculating the amount of opioid the patient is requiring over 24 hours; (2) converting to an equianalgesic dose of the chosen new analgesic (using equianalgesic dose tables: Table 1.32-2); (3) reducing the dose by 25% to 30% to account for cross-tolerance of opioids (the patient may have developed tolerance to the respiratory depressing effect of an opioid over time but may lose some of this tolerance when switching to the new opioid); and (4) dividing the total dose into a regular dosing schedule with an appropriate breakthrough (prn) dose. After an opioid rotation the patient needs to be monitored for analgesic and adverse effects. The dose will need to be titrated up or down to achieve the intended effect.
Opioid rotation is best carried out by a palliative care or pain clinician.
Adjuvant analgesics (coanalgesics) are medications that are not primarily classified as analgesics but do have analgesic properties in certain types of pain or enhance the effects of analgesics. When appropriate, coanalgesics should be used at all steps of the WHO analgesic ladder.
1. Antiepileptic drugs used as adjuvants for neuropathic pain: In the context of managing neuropathic pain in patients with advanced disease, gabapentin and pregabalin are now the most commonly used anticonvulsants. Newer anticonvulsants such as oxcarbazepine, lamotrigine, and topiramate do not appear to be more effective than gabapentin and pregabalin, while often being more expensive.
1) Gabapentin: The usual starting dose is 200 to 300 mg orally in the evening (100 mg orally in elderly patients and those with renal impairment), with the dose increased by 200 to 300 mg (100 mg/d in the elderly) every 2 to 3 days until an adequate analgesic effect is achieved or adverse effects develop. Patients with advanced disease and those receiving palliative care usually require oral doses <900 mg/d in total, usually in divided doses bid. Somnolence is the most common adverse effect that limits the dose and titration of the medication. Reduce the dose in patients with renal impairment.
2) Pregabalin: While not routinely used as an anticonvulsant, it has similar analgesic activity to gabapentin. The starting doses of 50 to 75 mg bid (25 mg bid in the elderly and those with renal impairment) can be titrated by 25 to 50 mg every 3 to 7 days. The maximum recommended effective dose is 300 mg bid, although patients with advanced disease seldom tolerate doses >300 mg/d. Total daily doses of 50 mg may be effective in patients with renal impairment and in the elderly. Common adverse effects include confusion and volume overload (from sodium and water retention).
3) Carbamazepine is the first-line therapy recommended for trigeminal neuralgia. The starting doses of 50 to 100 mg bid can be titrated to a maximum of 800 to 1200 mg/d. Lower doses are needed in those with renal impairment, frailty, advanced disease, and in the elderly. The main adverse effect is sedation, which can be managed with dose reduction. The effectiveness of analgesia is not related to drug levels (unlike when carbamazepine is used as an anticonvulsant). There is no need to regularly measure drug levels unless toxicity is suspected (based on the clinical situation or high drug doses).
2. Tricyclic antidepressants (TCAs): This class of medications has been shown to be effective in the management of neuropathic pain related to cancer, advanced organ diseases such as neurologic diseases, migraine, postherpetic neuralgia, fibromyalgia, and diabetic neuropathy. Adverse effects are largely related to anticholinergic effects (eg, sedation, dry mouth, constipation, urinary retention, and confusion) and often limit dosing.
1) Amitriptyline: Starting dose 10 mg at bedtime, increased to 25 mg after 3 to 7 days, and then increased by 25 mg/d every 1 to 2 weeks. Effective analgesia (or adverse effects) can often develop with doses <50 mg/d.
2) Nortriptyline: Starting dose 10 mg at bedtime, increased to 25 mg after 3 to 7 days, and then increased by 25 mg/d every 1 to 2 weeks. Effective analgesia (or adverse effects) can often develop with doses <75 mg/d.
3. Other antidepressants: Evidence suggests that selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs) have coanalgesic effects but may be slightly inferior to TCAs. However, the adverse effects of SSRIs and SNRIs are better tolerated by most patients, making these agents a suitable option to consider for the management of neuropathic pain.
Duloxetine is the only SNRI officially approved in Canada for the management of diabetic neuropathy, fibromyalgia, and chronic musculoskeletal pain. The starting dose of 30 mg is effective in some patients, whereas others need to have their dose increased to a maximum of 60 mg/d after 1 week of treatment with the starting dose. Duloxetine should not be used in patients with end-stage kidney disease or cirrhosis.
4. Other adjuvant analgesics: Many other agents are used as coanalgesics in specific situations:
1) Glucocorticoids (eg, dexamethasone) are a useful temporary adjuvant analgesic to help manage crises or severe acute exacerbations of somatic, visceral, or neuropathic pain. They provide pain relief in the setting of inflammatory conditions and when pain is caused by compression, stretch, or infiltration by a tumor. Long-term adverse effects of glucocorticoids are extensive. Their use should be limited to as short a duration of time as possible (a few days) unless the patient is actively dying, at which time the benefits of pain relief should be weighed against any active adverse effects before considering weaning or changing doses.
2) Bisphosphonates may help reduce metastatic bone pain related to extensive bone metastases (osteolytic or osteoblastic). However, to achieve this effect, they need to be administered regularly (usually monthly) by the parenteral route (pamidronate or zoledronic acid) for ≥3 months. They are therefore not indicated as primary analgesics or for managing acute bone pain.
As IV bisphosphonates have additional effects (eg, treatment of hypercalcemia, reduction of malignant skeletal events, reduced need for radiotherapy in malignancy), some cancer treatment protocols include pamidronate or zoledronic acid.
Oral bisphosphonates are commonly used in the prevention and treatment of osteoporosis. These formulations have not been shown to be effective as analgesia.
3) Topical lidocaine and capsaicin are effective in treating localized pain due to neuropathy, vasculitis, or trauma, but they are not generally effective in patients with advanced disease such as cancer and those with chronic neuropathic pain.
4) Ketamine administered by continuous subcutaneous infusion (for several days) and lidocaine given by short-term infusions (several hours) may be considered as third-line adjuvant analgesics in a small group of selected patients with severe neuropathic pain related to advanced cancer who have not responded to first-line (TCAs, anticonvulsants) or second-line (glucocorticoids) adjuvant analgesics and opioid titration. These should only be prescribed and used by palliative care or pain specialists.
5) Nerve blocks and neurodestructive procedures are highly specialized procedures in which anesthetic agents or alcohol are used to target a nerve plexus felt to be the source of pain in order to stop the transmission of pain signals. Anesthetic agents temporarily anesthetize a ganglion or nerve, whereas alcohol injections are used for more permanent destruction. These procedures are used uncommonly and only in few highly selected patients. The success rates are improved with imaging guidance. Not all clinics and hospitals are able to provide these interventions, as they require specialized skills.
a) Celiac plexus block or neurolysis may be used in the treatment of pain caused by pancreatic, gastric, liver, gallbladder, intestinal, or renal cancers; retroperitoneal metastases; or splenomegaly.
b) Hypogastric plexus neurolysis or blocks may be used in patients with pelvic pain secondary to malignant and nonmalignant causes, tenesmus secondary to radiation therapy to the rectum, or malignancy-related rectal pain.
c) Blocks of the stellate ganglion, lumbar sympathetic trunk, and ganglion impar (ganglion of Walther) can be used to ease pain in the limbs, face, and pelvic region.
d) Continuous central neuraxial blocks (subarachnoid and epidural) are most commonly performed when systemic administration of opioids is ineffective or results in unacceptable adverse effects. In these selected cases opioids are administered epidurally or intrathecally along with a local anesthetic agent (eg, bupivacaine). Indwelling catheters that are tunneled under the skin (to reduce the risk of infection) are used to provide continuous infusions of medications into these spaces. These blocks require special expertise and are reserved for patients with intractable neuropathic and nociceptive pain.
e) Peripheral nerve blocks are often used for procedures and interventions. A long-term anesthetic effect could be achieved through alcohol lysis of the same nerves.
f) Trigger point injections are injections of an anesthetic agent with or without a glucocorticoid into a muscle knot (trigger point). The exact mechanism of pain control is not clear but improvement in local pain has been shown in patients with myofascial pain and fibromyalgia. The injections are ineffective for the management of pain from cancer or advanced disease.
An in-depth analysis of nonpharmacologic interventions of pain management is beyond the scope of this chapter. However, in some situations, they can be very useful analgesic procedures. Patients with advanced disease and those at the end of life may not experience much benefit from nonpharmacologic therapies, as these modalities require active engagement and patients may not have the physical or cognitive stamina to participate fully.
1. Radiotherapy is the first-line treatment for localized pain associated with focal or metastatic cancer. It is usually administered as external therapy. Radiotherapy is very effective, leading to pain reduction in up to 75% of patients, with some experiencing total relief. When used for palliation (eg, with the goal defined not as cure but control of the disease or palliation of symptoms), it is administered in multiple or in single fractions (doses). Single fractions have been shown to be generally as effective as multiple doses and linked with similar adverse effects, which are uncommon when palliative doses are used.
2. Surgery can be a useful method of pain relief in selected patients. Prophylactic and postfracture bone fixation, for example, can provide rapid and effective pain control. Spinal decompression, vertebroplasty, and kyphoplasty have shown mixed results in the management of pain from degenerative disc disease, vertebral compression fractures, and metastatic vertebral disease.
3. Transcutaneous electrical nerve stimulation (TENS) uses electrical stimulation applied to the skin to stimulate nerve endings, in this way altering or eradicating pain. This form of analgesia is easily performed in the outpatient setting. Results are most beneficial when TENS is used for acute and subacute pain from injury or surgery. It is generally not used in the management of pain from cancer or advanced disease.
4. Physiotherapy, including massage and exercise, is most effective in the management of bone, soft tissue, and neuropathic pain. Physiotherapy is important in maintaining mobility despite ongoing pain and disease.
5. Occupational therapy does not directly provide analgesia. Through modifications in behavior, gait aids, and tools, occupational therapy can indirectly reduce pain through altered body mechanics and minimization of ongoing injury.
6. Cognitive behavioral therapy and behavior therapy have been shown to have small benefits in overcoming disability, managing mood, and reducing catastrophic thinking associated with chronic pain. It is not clear at this time how long the therapy should be continued for or which patients benefit most from such interventions. Benefit to those with advanced disease is limited due to the level of energy patients must exert to achieve results.
Tables and FiguresTop
|
Opioid |
Usual starting dose |
Frail persons, very elderly persons, patients with advanced heart and lung disease |
|
Codeine |
15 mg PO every 4 h + 15 mg PO every 2 h as neededa |
7.5 mg PO every 6 h + 7.5 mg PO every 2 h as neededa |
|
Tramadol |
25-50 mg PO every 8 h + 37.5 mg or 50 mg PO every 4 h as neededb |
25-37.5 mg PO bid + 37.5 mg PO every 4 h as neededb |
|
Morphine |
5 mg PO every 4 h + 5 mg PO every 1 h as needed |
1 mg or 2.5 mg PO every 6 or 8 h + 1 mg PO every 2 h as needed (select a dose; range provided here only to individualize dose to frailty level) |
|
Hydromorphone |
1 mg PO every 4 h + 1 mg PO every 2 h as needed |
0.2 mg PO or 0.5 mg PO every 6 or 8 h (select a dose; range provided here only to individualize dose to frailty level) + 0.5 mg PO every 2 h as needed |
|
Oxycodone |
2.5 mg or 5 mg PO every 4 h + 5 mg PO every 2 h as needed |
|
|
This table serves only as a general guideline; doses may sometimes need to be individualized. Avoid prescribing ranges of doses; choose one dose and assess its impact. If not effective, titrate up. Ranges are confusing for patients, caregivers, and care providers. In monitored settings, as-needed dosing can be done every 1 hour (as needed). Where there are concerns of inappropriate use of breakthrough doses, consider increasing the interval to every 3 or even 4 hours. |
||
|
a The maximum daily dose of codeine is ~300 mg PO in 24 h. b The maximum daily dose of tramadol is 400 mg PO in 24 h. |
||
|
Courtesy of Pallium Canada (www.pallium.ca), a nonprofit Canadian foundation that develops palliative care education programs. |
||
|
bid, twice daily; PO, oral. |
||
|
Drug |
PO equianalgesic dose |
To convert to OME,* multiply by: |
To convert from OME, multiply by: |
To convert from PO to SC or IVa,b (same drug) multiply by: |
|
Morphine |
5 mg |
− |
− |
0.5 |
|
Codeine |
50 mg |
0.1 |
10 |
0.5 (SC only; IV not available in Canada) |
|
Oxycodone |
2.5-3 mg |
1.5-2 |
0.75-0.5 |
Not available in Canada |
|
Hydromorphone |
1 mg |
5 |
0.2 |
0.5 |
|
Methadone |
Methadone is 4-10 times more potent than morphine (see text) and is available in Canada in oral form only. Conversions between methadone and morphine should be carried out by practitioners experienced in using both medications. |
|||
|
Tramadol |
Tramadol is ~8-10 times less potent than morphine. |
|||
|
Buprenorphine |
5 microg/h patch = 30 mg OME (total daily morphine dose) 10 microg/h patch = 30-80 mg OME (total daily morphine dose) Higher doses (35, 52.5, 70 microg/h) available in some countries |
|||
|
Fentanyl |
Transdermal fentanyl (patches) is a controlled-release formulation that should be used only in those already on an opioid and at a dose equivalent to ≥60 mg/d OME (24 h). Conversion of morphine to fentanyl: table 1.32-3 Parenteral formulations available for SC or IV administration |
|||
|
a SC dosing is limited to ≤2 mL per injection site. If concentration of the drug does not allow for this, >1 injection site or conversion to IV dose is required. b In Canada, the SC route is generally considered equianalgesic to the IV route (the equianalgesic dose ratio of PO to SC and IV is 2:1 for both morphine and hydromorphone). In Europe, the IV route is considered somewhat more potent than the SC route (the PO to SC equianalgesic dose ratio is 2:1 and the PO to IV ratio is considered 3:1). |
||||
|
Adapted from Michael G. DeGroote National Pain Centre. https://npc.healthsci.mcmaster.ca/guidelines/; Health Canada. Important Changes to the Dose Conversion Guidelines for Fentanyl Transdermal Systems - For Health Professionals. http://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2009/14548a-eng.php; and Centre for Effective Practice. Opioid Manager. https://cep.health/clinical-products/opioid-manager/. |
||||
|
IV, intravenous; OME, oral morphine equivalent; PO, oral; SC, subcutaneous. |
||||
|
Morphine PO (mg/d) |
Hydromorphone PO (mg/d) |
Oxycodone PO (mg/d) |
Fentanyl patch (microg/h) |
|
45-59 |
6-11 |
30-44 |
12d |
|
60-134 |
12-26 |
45-89 |
25 |
|
135-179 |
27-35 |
90-119 |
37 |
|
180-224 |
36-44 |
120-149 |
50 |
|
225-269 |
45-53 |
150-179 |
62 |
|
270-314 |
54-62 |
180-209 |
75 |
|
315-359 |
63-71 |
210-239 |
87 |
|
360-404 |
72-80 |
240-269 |
100 |
|
405-449 |
81-89 |
270-299 |
112 |
|
450-494 |
90-98 |
300-329 |
125 |
|
495-539 |
99-107 |
330-359 |
137 |
|
540-584 |
108-116 |
360-389 |
150 |
|
585-629 |
117-125 |
390-419 |
162 |
|
630-674 |
126-134 |
420-449 |
175 |
|
675-719 |
135-143 |
450-479 |
187 |
|
720-764 |
144-152 |
480-509 |
200 |
|
765-809 |
153-161 |
510-539 |
212 |
|
810-854 |
162-170 |
540-569 |
225 |
|
855-899 |
171-179 |
570-599 |
237 |
|
900-944 |
180-188 |
600-629 |
250 |
|
945-989 |
189-197 |
630-659 |
262 |
|
990-1034 |
198-206 |
660-689 |
275 |
|
1035-1079 |
207-215 |
690-719 |
287 |
|
1080-1124 |
216-224 |
720-749 |
300 |
|
How to use this table: Patients using an oral opiate in the dose range listed in the table can be carefully converted to transdermal fentanyl in the dose in the corresponding far right column. |
|||
|
a Initiation of fentanyl in patients who are opioid-naive is contraindicated at any dose. b The conversion table is unidirectional only and should only be used to convert adult patients from their current oral or parenteral opioid analgesics to the approximate fentanyl transdermal patch for use in chronic pain. c Do not convert patients previously on codeine or tramadol to fentanyl transdermal patch due to significant interpatient variability in metabolism, safety, and effectiveness of these drugs. d Health Canada recommends that 12 microg/h patches be used for dose titration or adjustments, not as the initiating dose. |
|||
|
Adapted from BC Cancer Agency. Palliative Care for the Patient with Incurable Cancer or Advanced Disease. Part 2: Pain and Symptom Management. Available at https://www2.gov.bc.ca/assets/gov/health/practitioner-pro/bc-guidelines/palliative2.pdf. Accessed September 13, 2022. |
|||
|
PO, oral. |
|||
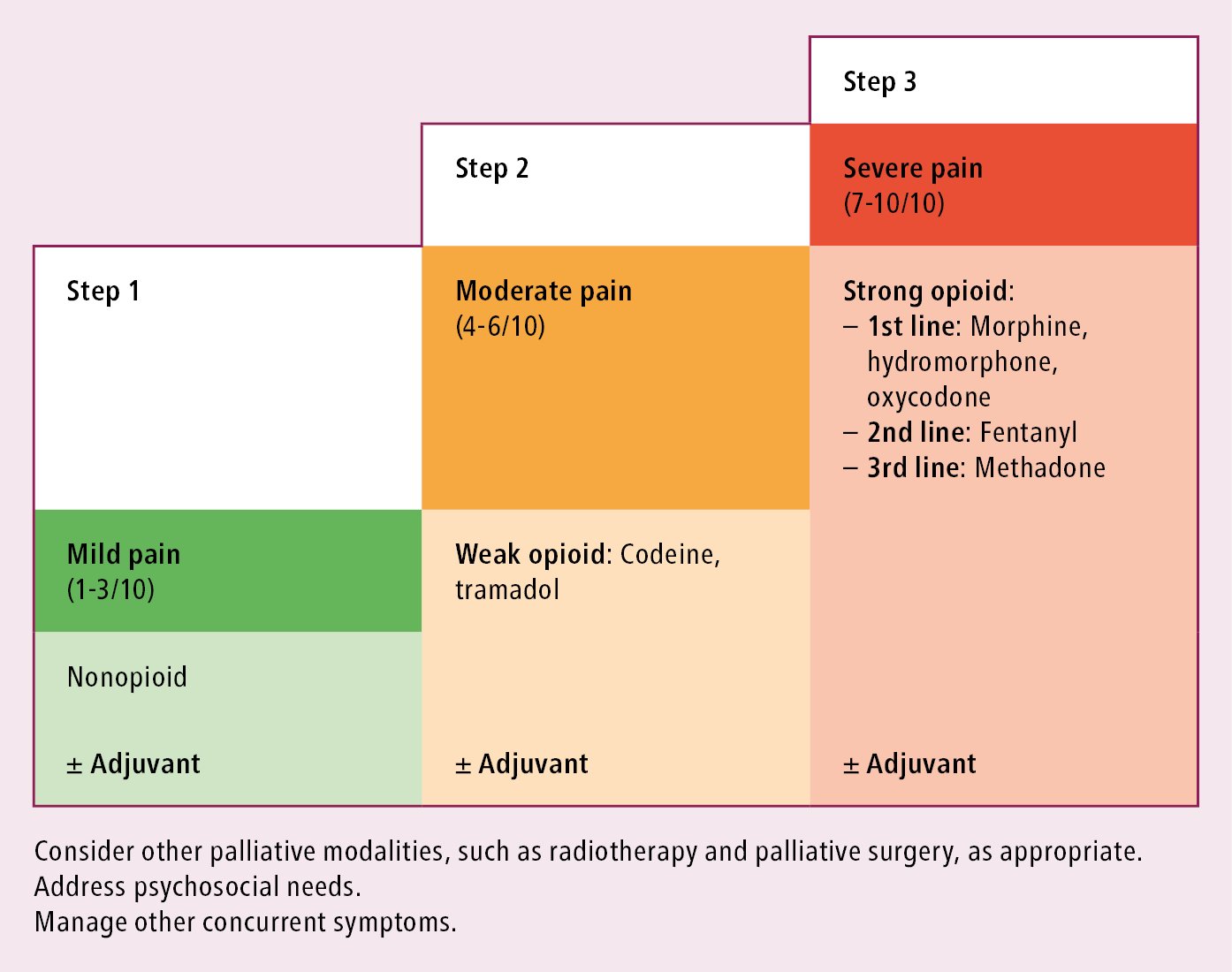
Figure 1.32-1. The World Health Organization analgesic ladder. Adopted from Pallium Canada (www.pallium.ca).