Sehgal K, Cifu AS, Khanna S. Treatment of Clostridioides difficile Infection. JAMA. 2022;328(9):881-882. doi: 10.1001/jama.2022.12251. PMID: 35939317.
Definition and EtiologyTop
1. Etiologic agents: Toxins A and B produced by Clostridioides difficile (CD), which is a spore-forming, anaerobic, gram-positive bacillus.
2. Reservoir and transmission: Soil, environment (especially hospitals, long-term care and daycare facilities), and individuals colonized with CD (5% of adults, 10%-25% of the older population, up to 50% of newborns and infants) are an important reservoir for environmental contamination, with or without underlying clinical infection. Transmission occurs via the fecal-oral route of ingesting the spore form of the organism.
3. Risk factors: Significant risk factors for Clostridioides difficile infection (CDI) include advanced age and current or recent (usually up to 3 months prior to disease onset, with the highest risk in the first month and then declining) antibiotic treatment, especially with clindamycin, cephalosporins, broad-spectrum penicillins, or fluoroquinolones. Narrow-spectrum beta-lactams, carbapenems, trimethoprim/sulfamethoxazole, and macrolides carry a medium risk. Vancomycin, linezolid, nitrofurantoin, metronidazole, tetracyclines/doxycycline, daptomycin, and tigecycline carry less risk.
Some other risk factors include gastrointestinal (GI) surgery, chemotherapy, inflammatory bowel disease (IBD), and gastric acid suppression (proton pump inhibitors [PPIs] and H2-receptor antagonists).
4. Recurrence of infection: Recurrence of CDI is defined as complete resolution followed by the subsequent development of CDI within 8 weeks of a previous episode. Up to 20% to 30% of patients experience CDI within 30 days of treatment completion. Following ≥2 recurrences, the risk of further episodes and more severe illness increases with each subsequent CDI episode.
Clinical Features and Natural HistoryTop
The clinical presentation of CDI ranges from mild diarrhea to fulminant colitis with septic shock.
The dominant clinical feature is diarrhea of varying intensity, ranging from 3 loose stools to >10 watery stools in a 24-hour period; fresh blood may rarely be present in stools. Lower abdominal cramping and fever may also be present. In severe cases patients may develop dehydration, ileus, and shock. Approximately 15% of patients with a milder form of CDI recover spontaneously, particularly when the causative antibiotic is discontinued. Patients with severe CDI may develop ileus of the colon or toxic megacolon (see Ulcerative Colitis) with minimal to no diarrhea.
Severe complicated CDI (defined as CDI leading to intensive care unit [ICU] admission), colectomy, or death occurs in 3% to 20% of patients. The mortality rate is generally reported as between 3% and 10% among those with severe disease. The death rate is higher in critically ill patients or in those with advanced age and multiple comorbidities, toxic megacolon, and/or colectomy, where the mortality rate can exceed 20%. Some indicators of more severe disease include high-grade fever (38.5 degrees Celsius), elevated peripheral white blood cell count (>15×109/L), low serum albumin (<30 g/L), and increased serum creatinine (a 50% increase from the premorbid level or levels ≥133 micromol/L).
The initial evaluation of disease severity and preconfirmation empiric management: Figure 7.2-1.
DiagnosisTop
1. Laboratory assays: Establishing the diagnosis of CDI requires the detection of toxins or a toxigenic strain of CD in a liquid stool sample confirmed by enzyme immunoassay (EIA) testing or nucleic acid amplification test (NAAT). Only stool samples from symptomatic patients should be sent for laboratory testing, as the current laboratory assays, especially the NAAT, cannot distinguish between CDI and asymptomatic CD colonization with absolute certainty. Since no single test has sufficiently high negative and positive predictive values, the results of the laboratory tests must be interpreted in conjunction with the clinical state of the patient. Approximate performance characteristics of currently available tests: Table 7.2-6.
One approach is to use an algorithm of screening a stool sample with a sensitive test (eg, EIA for the detection of the common antigen glutamate dehydrogenase [GDH]) or NAAT and, if positive, proceed to test the sample with more specific EIA testing to detect free toxin(s). Another approach is to perform both sensitive and specific tests simultaneously (eg, EIA for GDH and free toxins) and interpret the results based on the current clinical status of the patient to avoid treating a colonized patient without infection. Toxigenic culture of the stool sample may provide additional information.
2. Colonoscopy may show pseudomembranous colitis, which is an inflammation of the colon characterized by typical yellow-grey plaques on the mucosa of the large intestine (pseudomembranes) ranging from a few millimeters to 1 to 2 centimeters in diameter (Figure 7.2-2). Pseudomembranes may not be apparent in patients with immunodeficiency and can be seen in other conditions such as cytomegalovirus colitis, other GI infections, inflammatory bowel disease or ischemic colitis.
The diagnosis of CDI consists of a combination of clinical symptoms and one of the following criteria:
1) Colonoscopic or histopathologic evidence of pseudomembranous colitis.
2) A stool sample positive for free CD toxins or toxigenic CD strain, including detection of the toxin gene by NAAT.
Diarrhea of another etiology (see Diarrhea); this diagnosis can be confirmed by medical history and microbiology test results. Ulcerative colitis may be differentiated from pseudomembranous colitis caused by CDI based on the persistent presence of fresh blood in stools, lack of laboratory evidence of CDI, and (predominantly) distinct microscopic appearance of mucosal lesions.
TreatmentTop
1. Mild CDI: If possible, discontinue the antimicrobial agent causing diarrhea. If continued treatment of the primary infection is necessary, switch to an alternative antibiotic that is less associated with CDI, as deemed clinically appropriate.
2. More severe CDI: Hospitalization is necessary for administration of IV fluids, correction of electrolyte disturbances, and monitoring for complications.
Treatment is not recommended for patients who have a positive laboratory test result but do not exhibit diarrhea or other signs of CDI, as this indicates asymptomatic carriage. Treatment is indicated for patients with typical manifestations of CDI, with acute diarrhea (≥3 loose stools within 24 h) with no obvious alternative explanation and a positive laboratory test. Empiric treatment should be strongly considered in patients with a high clinical suspicion of CDI, particularly those with symptoms suggestive of severe or fulminant colitis, while awaiting the laboratory test results of diagnostic testing.
1. The initial episode or first recurrence of severe CDI, including pseudomembranous colitis:
1) Oral vancomycin 125 mg qid for 10 days; this is used in patients with severe CDI and in pregnant or breastfeeding women. In patients with fulminant colitis or toxic megacolon, administer IV metronidazole 500 mg every 8 hours combined with vancomycin 500 mg qid given orally or via nasogastric tube. When delivery of oral vancomycin to the colon is uncertain, it may also be delivered as a 30- to 60-minute retention enema: 500 mg in 100 mL of normal saline every 6 hours combined with IV metronidazole 500 mg tid.
2) Oral fidaxomicin 200 mg bid for 10 days can also be used. The rate of clinical cure is similar between vancomycin and fidaxomicin, but fidaxomicin may reduce the recurrence rate by 50% compared with vancomycin for non–North American Pulsed Field type 1 (NAP1) strains.Evidence 1Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due to imprecision. Louie TJ, Miller MA, Mullane KM, et al; OPT-80-003 Clinical Study Group. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011 Feb 3;364(5):422-31. doi: 10.1056/NEJMoa0910812. PMID: 21288078.
3) Oral metronidazole 500 mg tid for 10 days for mild to moderate disease (without features of severe disease). The cost of oral metronidazole is usually significantly lower than that of vancomycin or fidaxomicin, with an ~10% absolute increase of the failure rate.Evidence 2Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due to risk of bias. Nelson RL, Suda KJ, Evans CT. Antibiotic treatment for Clostridium difficile‐associated diarrhoea in adults. Cochrane Database of Systematic Reviews 2017, Issue 3. Art. No.: CD004610. DOI: 10.1002/14651858.CD004610.pub5. PMID: 28257555; PMCID: PMC6464548.
2. Fulminant CDI with hypotension, ileus, or megacolon: Vancomycin 500 mg qid orally or via nasogastric tube or 500 mg rectally in 100 mL normal saline every 6 hours combined with IV metronidazole 500 mg every 8 hours.
3. Second episode of CDI or subsequent recurrent CDI: If metronidazole alone was used to treat the first episode, oral vancomycin 125 mg qid for 10 days or fidaxomicin 200 mg bid for 10 days could be used. If a 10-day course of vancomycin was used for the previous episode, use vancomycin as a prolonged tapered regimen (eg, 125 mg given orally qid for 7 days; 125 mg given orally bid for a week; 125 mg given orally once daily for a week and then every 2 or 3 days for 2-8 weeks) based on current guidelines.
Approximately 50% of patients with multiple recurrent CDI respond to a tapering vancomycin regimen. An alternative to antibiotic therapy for recurrent CDI is fecal microbiota transplant (FMT), which replenishes the protective colonic microbiota to prevent future episodes of CDI. Patients who have had ≥2 recurrences of CDI and received ≥1 course of a tapering vancomycin regimen may benefit from FMT.
In patients with fulminant CDI, colectomy can be a life-saving procedure if performed at an early stage of fulminant colitis. Laboratory parameters suggestive of fulminant CDI include peripheral white blood cell counts >50×109 cells/L and serum lactate levels >5.0 mmol/L. The usual practice is subtotal colectomy. However, a retrospective study comparing subtotal colectomy to a colon-sparing procedure of diverting loop ileostomy showed a mortality benefit with loop ileostomy at 19% versus 50% in historical controls and preservation of the colon in >90% of patients.Evidence 3Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due to the observational nature of data but increased to moderate due to effect size. Neal MD, Alverdy JC, Hall DE, Simmons RL, Zuckerbraun BS. Diverting loop ileostomy and colonic lavage: an alternative to total abdominal colectomy for the treatment of severe, complicated Clostridium difficile associated disease. Ann Surg. 2011 Sep;254(3):423-7; discussion 427-9. doi: 10.1097/SLA.0b013e31822ade48. PubMed PMID: 21865943.
Follow-UpTop
Treatment may be considered effective when fever resolves within 24 to 48 hours and diarrhea resolves by 4 to 5 days of treatment. Follow-up testing for CD toxins should not be done to determine cure, as there is no laboratory test of cure. Patients should be educated about the potential for CDI recurrence to avoid complications due to delay in seeking medical attention. CDI recurrence usually occurs 1 to 2 weeks following completion of CDI treatment, but rarely patients can experience recurrence 1 to 2 months after completing treatment. Patients with suspected CDI recurrence should be managed according to the clinical presentation.
ComplicationsTop
Complications of CDI include fulminant colitis and toxic megacolon (see Ulcerative Colitis), adynamic ileus, colonic perforation and peritonitis, and edema due to hypoalbuminemia caused by intestinal protein loss.
PreventionTop
Judicious use of antimicrobial agents.
Frequent hand washing with soap and water whenever possible.
In hospitals, long-term medical care institutions, and daycare facilities it is necessary to:
1) Follow strict hand hygiene rules (washing hands with soap and water whenever possible).
2) Isolate and implement contact precautions for CDI patients.
3) Wear gown and disposable gloves when caring for CDI patients.
4) Use designated equipment and adhere to proper disposition of soiled diapers.
5) Use CD sporicidal agents to disinfect rooms and bathrooms.
Tables and FiguresTop
|
Test |
Target |
Sensitivity |
Specificity |
NPV |
PPV |
Turnaround time |
|
EIA |
Toxins A + B |
60% |
98% |
Low |
High |
20-90 min |
|
GDH |
Common antigen |
90% |
50% |
High |
Low |
20-90 min |
|
NAAT (PCR, LAMP) |
Toxin B gene |
90% |
65% |
High |
Low |
90-200 min |
|
GDH, glutamate dehydrogenase; LAMP, loop-mediated isothermal amplification; NAAT, nucleic acid amplification test; NPV, negative predictive value; PCR, polymerase chain reaction; PPV, positive predictive value. |
||||||
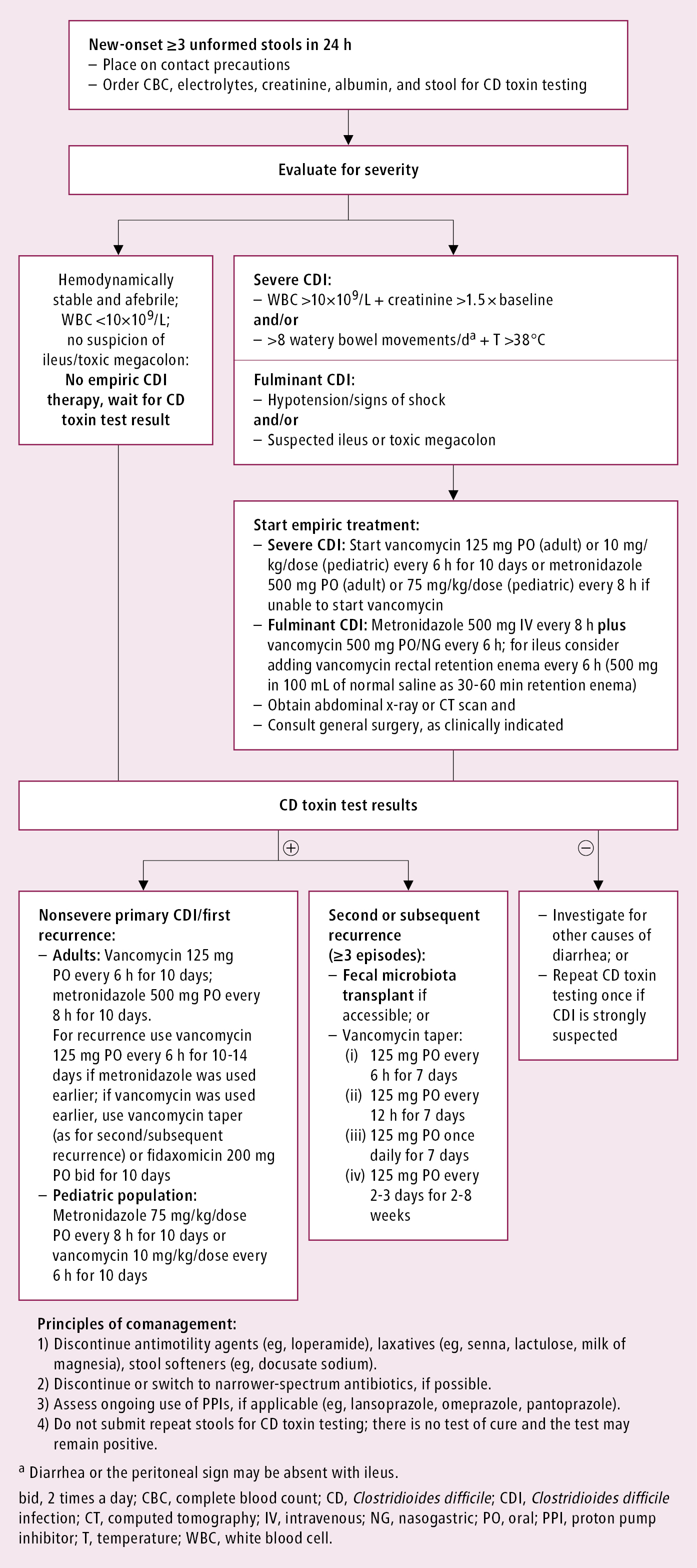
Figure 7.2-1. The initial evaluation of disease severity and preconfirmation empiric management in Clostridioides difficile infection.
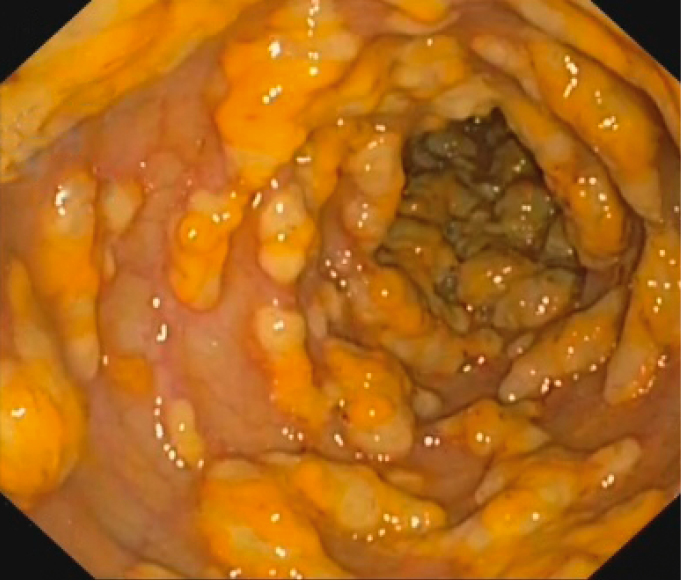
Figure 7.2-2. Endoscopic appearance of pseudomembranous colitis.