Alali AA, Pittayanon R, Martel M, et al. TC-325 Superiority in Malignant Gastrointestinal Bleeding: An Individual Patient Data Meta-Analysis of Randomized Trials. Am J Gastroenterol. 2024 Sep 9. doi: 10.14309/ajg.0000000000003078. Epub ahead of print. PMID: 39248610.
Carson JL, Stanworth SJ, Dennis JA, et al. Transfusion thresholds for guiding red blood cell transfusion. Cochrane Database Syst Rev. 2021 Dec 21;12(12):CD002042. doi: 10.1002/14651858.CD002042.pub5. PMID: 34932836; PMCID: PMC8691808.
Barkun AN, Almadi M, Kuipers EJ, et al. Management of Nonvariceal Upper Gastrointestinal Bleeding: Guideline Recommendations From the International Consensus Group. Ann Intern Med. 2019 Dec 3;171(11):805-822. doi: 10.7326/M19-1795. Epub 2019 Oct 22. PMID: 31634917; PMCID: PMC7233308.
Oakland K, Chadwick G, East JE, et al. Diagnosis and management of acute lower gastrointestinal bleeding: guidelines from the British Society of Gastroenterology. Gut. 2019 May;68(5):776-789. doi: 10.1136/gutjnl-2018-317807. Epub 2019 Feb 12. PMID: 30792244.
Stanley AJ, Laine L. Management of acute upper gastrointestinal bleeding. BMJ. 2019 Mar 25;364:l536. doi: 10.1136/bmj.l536. PMID: 30910853.
Strate LL, Gralnek IM. ACG Clinical Guideline: Management of Patients With Acute Lower Gastrointestinal Bleeding. Am J Gastroenterol. 2016 Apr;111(4):459-74. doi: 10.1038/ajg.2016.41. Epub 2016 Mar 1. Erratum in: Am J Gastroenterol. 2016 May;111(5):755. PMID: 26925883; PMCID: PMC5099081.
Gerson LB, Fidler JL, Cave DR, Leighton JA. ACG Clinical Guideline: Diagnosis and Management of Small Bowel Bleeding. Am J Gastroenterol. 2015 Sep;110(9):1265-87; quiz 1288. doi: 10.1038/ajg.2015.246. Epub 2015 Aug 25. PMID: 26303132.
RÃos Castellanos E, Seron P, Gisbert JP, Bonfill Cosp X. Endoscopic injection of cyanoacrylate glue versus other endoscopic procedures for acute bleeding gastric varices in people with portal hypertension. Cochrane Database Syst Rev. 2015 May 12;(5):CD010180. doi: 10.1002/14651858.CD010180.pub2. Review. PMID: 25966446.
Sreedharan A, Martin J, Leontiadis GI, et al. Proton pump inhibitor treatment initiated prior to endoscopic diagnosis in upper gastrointestinal bleeding. Cochrane Database Syst Rev. 2010 Jul 7;(7):CD005415. doi: 10.1002/14651858.CD005415.pub3. Review. PMID: 20614440.
Definition, Etiology, PathogenesisTop
Gastrointestinal (GI) bleeding may be divided into:
1) Upper GI bleeding (from a source located above the ligament of Treitz), which occurs in ~80% of patients hospitalized due to GI bleeding. The most common causes include gastric or duodenal ulcers, acute hemorrhagic (erosive) gastropathy, esophageal varices, Mallory-Weiss syndrome, and esophagitis; less common causes include Dieulafoy lesions, duodenitis, neoplasms, esophageal ulcers, and vascular malformations. Acute upper GI bleeding may be precipitated by shock, systemic inflammatory response syndrome, sepsis, multiple trauma, acute respiratory failure, multiorgan failure, severe burns, and other acute and severe diseases.
2) Lower GI bleeding (from a source located below the ligament of Treitz), which occurs in ~20% of patients hospitalized due to GI bleeding. The most common causes of severe lower GI bleeding in adults are colonic diverticula and vascular malformations, while the less common causes include inflammatory bowel disease (IBD), bowel ischemia, postradiation colitis (after radiotherapy for prostate or female reproductive tract cancers), angiodysplasia, hemorrhoidal disease, and neoplasms. In children and adolescents the causes may include intussusception (secondary to polyps), IBD, Meckel diverticular bleed, and small intestinal or colonic polyps.
Bleeding may be exacerbated by coagulopathy.
GI bleeding can be also divided into:
1) Overt GI bleeding: Bleeding that is visible, such as hematemesis (bloody or coffee-ground emesis), hematochezia (presence of blood and blood clots in feces), or melena (black tarry stools). It may be overt-obscure if investigations do not establish the exact source.
2) Occult GI bleeding: Bleeding which is not overt (eg, detected through occult blood testing or confirmed only after investigations for iron-deficiency anemia). It may also be occult-obscure.
Clinical FeaturesTop
1. Acute bleeding:
1) Upper GI bleeding: The most common presentation is melena (tarry, black, foul-smelling stools). If the bleeding is brisk, hematochezia or maroon stools may be observed. Sometimes the presenting symptom is hematemesis with bright red blood or coffee-ground emesis. Epigastric or diffuse abdominal pain may be present (chest pain mimicking coronary disease occurs in some patients) as well as symptoms of hypovolemia (potentially leading to shock).
2) Lower GI bleeding: Hematochezia is the most common presentation, but ~10% of lower GI bleeding presents with melena. Symptoms of hypovolemia may occur (potentially leading to shock).
2. Chronic bleeding may be asymptomatic or present as intermittent blood in stool, iron deficiency anemia, or fecal occult blood. Patients often have obscure GI bleeding (bleeding from an unknown source despite investigations including upper and lower GI endoscopy and small bowel radiography).
DiagnosisTop
History may indicate the source of bleeding, but usually diagnosis can only be established on the basis of endoscopy or, in the case of a massive hemorrhage, during radiologic intervention or surgery.
1. Laboratory tests:
1) Complete blood count (CBC): A decrease in hematocrit, hemoglobin levels, and red blood cell (RBC) counts may not be evident until blood becomes diluted by the transition of the intercellular fluid into the intravascular space, which may take hours, or by infused fluids that contain no blood cells (eg, 0.9% NaCl).
2) International normalized ratio (INR) and other coagulation tests: These are particularly important in patients treated with anticoagulants, such as vitamin K antagonists, especially because information concerning such treatment sometimes cannot be obtained from individuals with an altered mental status. Coagulopathy may be also indicative of liver dysfunction or depletion of coagulation factors. Recent use of direct oral anticoagulants (DOACs) may require more specialized input (see Anticoagulant Agents).
3) Fecal occult blood tests (FOBTs) have no role in evaluation of the presence or localization of suspected GI bleeding, including evaluation for iron deficiency anemia.
2. Endoscopy of the upper GI tract is the key diagnostic and often therapeutic modality for suspected upper GI bleeding. The most common lesions identified on upper endoscopy are peptic ulcers.
Ulcers are generally characterized by the Forrest classification, which reliably predicts the likelihood of rebleeding:
– Class I: Active spurting bleeding (Ia) or active oozing bleeding (Ib).
– Class IIa: Visible nonbleeding vessel.
– Class IIb: Adherent clot at the ulcer base.
– Class IIc: Flat pigmented spot at the ulcer base.
– Class III: Clean (white) ulcer base.
Classes Ia, Ib, and IIa are associated with a high risk of rebleeding and are usually treated endoscopically in combination with injection therapy, coagulation, or clips. Class IIb lesions warrant targeted irrigation to attempt to dislodge the clot and then treat the underlying lesion. Classes IIc and III usually do not require endoscopic therapy, as they are associated with a low risk of rebleeding.
The urgency of endoscopy depends on the clinical situation and is variably described as up to 24 hours from presentation (possibly much sooner in unstable patients). If urgent/early endoscopy is not feasible, introduce a nasogastric tube (after securing the airway if needed). The presence of bile without blood in the gastric aspirate suggests a lower GI source of bleeding.
Of note, urgent upper GI endoscopy may be indicated in patients who are unstable with hematochezia, as it is prudent to exclude a briskly bleeding upper GI source.
3. Colonoscopy is the initial diagnostic procedure for patients with lower GI bleeding. It should be performed after adequate bowel preparation, with timing depending on the clinical presentation and perceived urgency (ideally within 24-48 h of presentation).
4. Computed tomography angiography (CTA) may be helpful in locating the source of active bleeding. These images may then guide catheter angiography, which can be used to embolize the bleeding vessel.
5. Some patients with obscure GI bleeding (usually after repeat endoscopy and/or colonoscopy) may require small bowel evaluations with enteroscopy (push or double-balloon) or wireless video capsule endoscopy (VCE); each may be combined with CT enterography.
TreatmentTop
Management algorithm for nonvariceal upper GI bleeding: Figure 7.2-1.
Management algorithm for bleeding varices: Figure 7.2-2.
1. Patients should be treated in an acute setting and then transferred to a general ward or monitored setting depending on the severity of their condition and clinical progress. In patients with significant blood loss and impaired mental status, maintain the airway and intubate if necessary.
2. Measure blood pressure. If it is normal, perform the measurement in a standing position to check for orthostatic hypotension. A decrease in systolic blood pressure >20 mm Hg, decrease in diastolic blood pressure >10 mm Hg, or increase in heart rate >30 beats/min may indicate significant volume depletion, although by itself this is neither sensitive nor specific. Check for signs of hypoperfusion, such as delayed capillary refill and other symptoms of shock. If needed, start treatment of shock (see Hemorrhagic Shock).
3. Correct blood volume for the blood lost due to the bleeding: Insert 2 large-bore peripheral vein catheters or a central venous catheter. Crystalloid is usually administered first as it is readily available. Packed RBCs (PRBCs) should be administered as soon as possible in patients with signs of hemodynamic instability or shock. Principles of large-volume transfusion include combination RBC and clotting agent preparations (eg, 1 unit of fresh frozen plasma for each 2 units of PRBCs) (see Hemorrhagic Shock). For more stable patients, transfusion of RBCs should be given to target a hemoglobin level of 7 to 8 g/dL.
4. Perform urgent/early endoscopy and attempt to stop the bleeding using local injections of vasoconstrictors (such as epinephrine) or saline, electrocautery, argon plasma coagulation, hemostatic clips, ligation of esophageal varices, and TC-325 (hemostatic powder spray) for malignant bleeds. The meaning of urgent/early endoscopy may depend on evolution of the patient’s clinical status and local availability of resources, but it likely falls within ≤24 hours if dictated by the level of clinical instability.
5. Start pharmacologic treatment:
1) In patients with high-risk bleeding gastric or duodenal ulcers or acute hemorrhagic gastropathy, administer an IV proton pump inhibitor (PPI) (eg, esomeprazole, omeprazole, or pantoprazole). Our pattern of practice is to administer a bolus injection followed by a continuous infusion or twice-daily dosing for 3 days (this also includes patients in whom bleeding was successfully stopped using endoscopy). If no further bleeding has occurred after 3 days of monitoring, the patient can be switched to an oral PPI. Low-risk lesions can be managed with initial oral PPI therapy. Of note, there is insufficient evidence to reach a firm conclusion about the superiority or noninferiority of PPI regimens for acute ulcer bleeding based on route (oral vs IV) and dose (high vs medium or low) of PPIs.Evidence 1Low Quality of Evidence (low confidence that we know true effects of the intervention). Quality of Evidence lowered due to risk of bias and imprecision. Neumann I, Letelier LM, Rada G, et al. Comparison of different regimens of proton pump inhibitors for acute peptic ulcer bleeding. Cochrane Database Syst Rev. 2013 Jun 12;(6):CD007999. doi: 10.1002/14651858.CD007999.pub2. Review. PMID: 23760821. All patients with peptic ulcers should be tested and, if positive, treated for Helicobacter pylori infection. Nonsteroidal anti-inflammatory drugs (NSAIDs) should be avoided (see point 4 below). The use of tranexamic acid is unlikely to be beneficial and is associated with an increased risk of venous thromboembolism.Evidence 2Weak recommendation (downsides likely outweigh benefits, but the balance is close or uncertain; an alternative course of action may be better for some patients). Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due to imprecision. HALT-IT Trial Collaborators. Effects of a high-dose 24-h infusion of tranexamic acid on death and thromboembolic events in patients with acute gastrointestinal bleeding (HALT-IT): an international randomised, double-blind, placebo-controlled trial. Lancet. 2020 Jun 20;395(10241):1927-1936. doi: 10.1016/S0140-6736(20)30848-5. PMID: 32563378; PMCID: PMC7306161.
2) In patients with bleeding esophageal varices, administer one of the following IV agents to lower portal pressure:
a) Terlipressin, a synthetic analogue of vasopressin with fewer adverse effects: 5 to 20 microg/min in a 20- to 40-minute infusion; if necessary, repeat the infusion every 8 hours for a maximum of 5 days. Alternatively injections of 1 to 2 mg every 4 to 6 hours may be used.
b) Somatostatin: 250 microg injection followed by a continuous infusion of 250 microg/h for 5 days.
c) Octreotide: 50 microg injection followed by a continuous infusion of 50 microg/h for 5 days.
3) Patients with cirrhosis and GI bleeding should receive antibiotics (eg, ceftriaxone for 1 week; switch to oral ciprofloxacin is possible if the patient is discharged earlier), as this is associated with a mortality benefit.Evidence 3Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due to the risk of bias. Chavez-Tapia NC, Barrientos-Gutierrez T, Tellez-Avila F, et al. Meta-analysis: antibiotic prophylaxis for cirrhotic patients with upper gastrointestinal bleeding - an updated Cochrane review. Aliment Pharmacol Ther. 2011 Sep;34(5):509-18. doi: 10.1111/j.1365-2036.2011.04746.x. Epub 2011 Jun 27. PMID: 21707680.
4) In patients treated with anticoagulants, neutralize the anticoagulant effects (vitamin K antagonists: see Table 3.1-4; heparin: see Heparins; fibrinolytic agents: see ST-Segment Elevation Myocardial Infarction). However, this should not delay endoscopy once needed. Hematology consultation may be required in case of use of DOACs. Also see Perioperative Direct Oral Anticoagulant Management. The management of both anticoagulants and antiplatelet drugs requires judgment of the risks and benefits of competing objectives and may require a multidisciplinary approach. The more efficacious the therapy of bleeding, the lower the risk of recurrent bleeding, and the higher the thromboembolic risk, the earlier resumption of the drugs is possible. Antithrombotic or antiplatelet drugs can usually be restarted, possibly with gradually increasing intensity, within 3 to 7 days of acute bleeding. Long-term PPI use may be required if the source of bleeding is relevant.
6. In patients with bleeding esophageal varices in whom endoscopic attempts to stop the bleeding have failed, you may introduce a Sengstaken-Blakemore tube into the esophagus and stomach or another type of tube (eg, Linton-Nachlas) allowing for balloon compression of the varices; the tubes should be removed within 24 hours to prevent pressure ulceration. The next step may involve shunting procedures (see below).
7. If endoscopic and pharmacologic treatment is ineffective, proceed with visceral angiography and selective embolization of the bleeding vessel or administration of terlipressin into the visceral vessels.
8. Since patients may require emergency surgery, involve a surgeon in the management at early stages of treatment. Surgery may be indicated in uncontrolled massive bleeding (ie, causing uncontrolled hemodynamic instability), also after an endoscopic or angiographic attempt to stop the bleeding; recurrent bleeding (following 2 endoscopic procedures); prolonged bleeding combined with an estimated blood loss >50% of circulating volume; and repeated hospitalizations due to a bleeding ulcer. The following surgical procedures are used:
1) Bleeding duodenal ulcer: Usually pyloroplasty or antrectomy are performed, both with oversewing of the bleeding ulcer. Vagotomy was historically performed as well, but it is now generally avoided as effective pharmacologic suppression of gastric acid is available.
2) Bleeding gastric ulcer or gastric erosions: Various types of resection, depending on the clinical situation and patient’s condition.
3) Bleeding esophageal varices unresponsive to endoscopic treatment, pharmacotherapy, and insertion of tubes compressing varices: Transvenous intrahepatic portosystemic shunt (TIPS) is a minimally invasive method. If this is ineffective, perform portosystemic anastomosis or revascularization and transection (dissection and suturing) of the esophagus as well as splenectomy.
4) Lower GI bleeding: Colonoscopy performed during surgery and guided by the operating surgeon sometimes enables localization of the source of bleeding. If this is successful, segmental intestinal resection with anastomosis is performed; if the source of bleeding in the colon cannot be located, colectomy with ileorectal anastomosis is performed.
Follow-UpTop
Routine follow-up gastroscopy ≤24 hours is not recommended in all patients treated endoscopically. It may be considered in patients at a high risk of recurrent bleeding, if the mucosa is not fully assessed due to residual blood, the source of the bleeding is not found, or there is uncertainty about the effectiveness of hemostasis. If the source of bleeding was a gastric ulcer, it is necessary to perform a control gastroscopy after ~4 weeks from the first examination.
FiguresTop
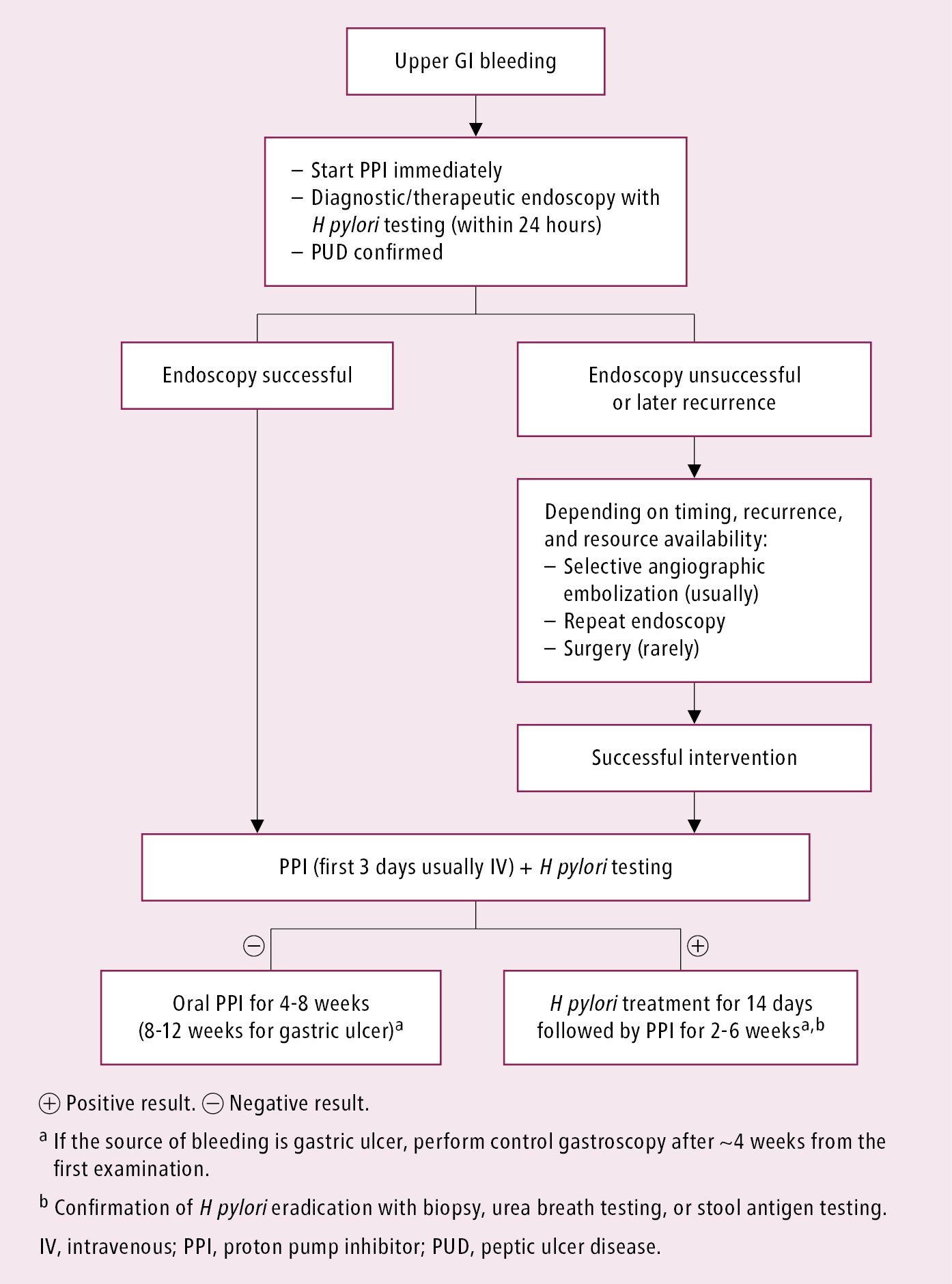
Figure 7.2-1. Management algorithm for nonvariceal upper gastrointestinal bleeding.
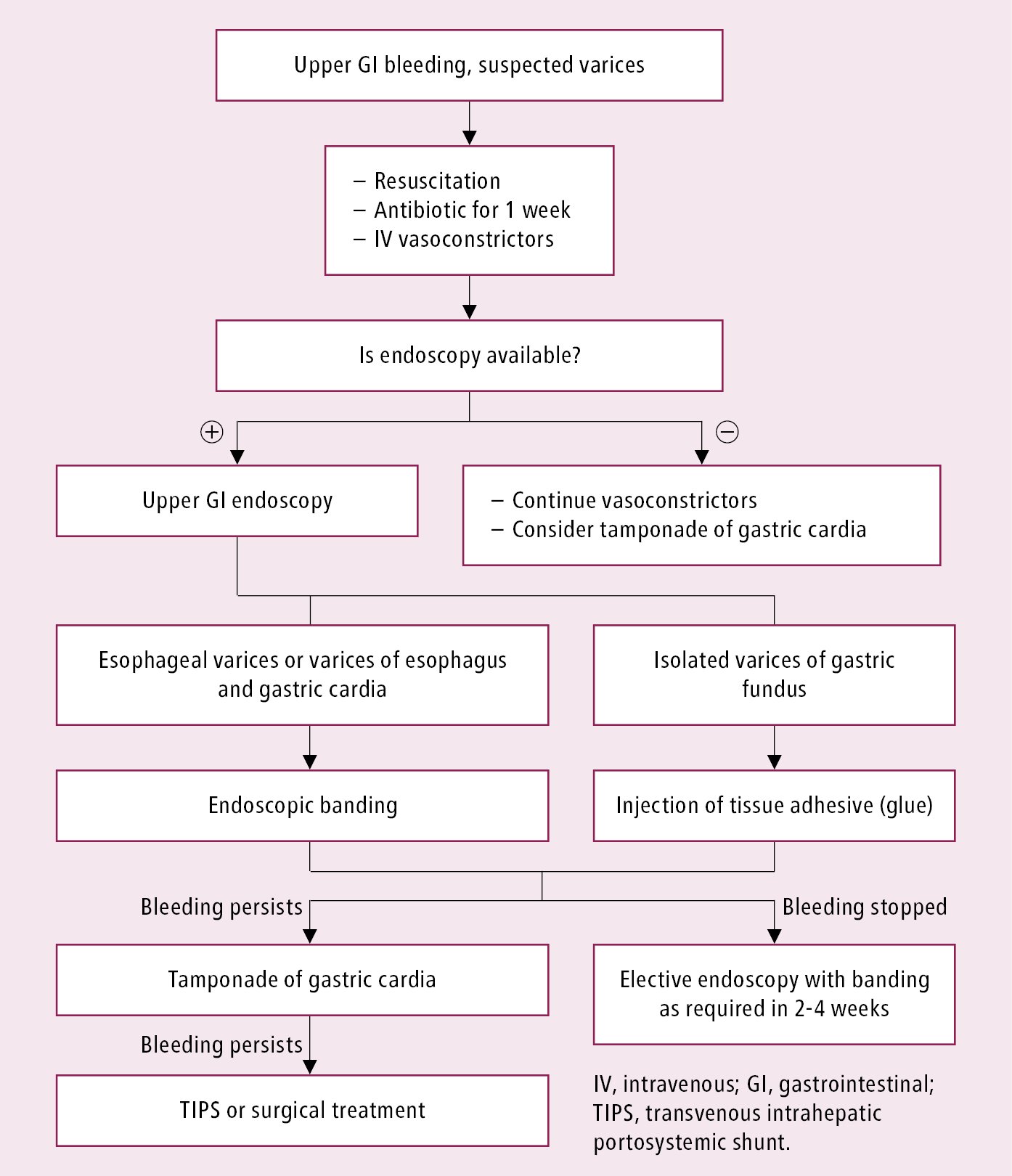
Figure 7.2-2. Management algorithm for bleeding esophageal varices.