Fitzpatrick KE, van den Akker T, Bloemenkamp KWM, et al. Risk factors, management, and outcomes of amniotic fluid embolism: A multicountry, population-based cohort and nested case-control study. PLoS Med. 2019 Nov 12;16(11):e1002962. doi: 10.1371/journal.pmed.1002962. PMID: 31714909; PMCID: PMC6850527.
Bernstein SN, Cudemus-Deseda GA, Ortiz VE, Goodman A, Jassar AS. Case 33-2019: A 35-Year-Old Woman with Cardiopulmonary Arrest during Cesarean Section. N Engl J Med. 2019 Oct 24;381(17):1664-1673. doi: 10.1056/NEJMcpc1904046. PMID: 31644848.
Extracorporeal Life Support Organization. General Guidelines for all ECLS Cases. August 2017. Version 1.4. Published August 2017. Accessed May 2020. https://www.elso.org/Resources/Guidelines.aspx
Clark SL, Romero R, Dildy GA, et al. Proposed diagnostic criteria for the case definition of amniotic fluid embolism in research studies. Am J Obstet Gynecol. 2016 Oct;215(4):408-12. doi: 10.1016/j.ajog.2016.06.037. Epub 2016 Jun 29. PMID: 27372270; PMCID: PMC5072279.
Jeejeebhoy FM, Zelop CM, Lipman S, et al; American Heart Association Emergency Cardiovascular Care Committee, Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation, Council on Cardiovascular Diseases in the Young, and Council on Clinical Cardiology. Cardiac Arrest in Pregnancy: A Scientific Statement From the American Heart Association. Circulation. 2015 Nov 3;132(18):1747-73. doi: 10.1161/CIR.0000000000000300. Epub 2015 Oct 6. PMID: 26443610.
Vanden Hoek TL, Morrison LJ, Shuster M, et al. Part 12: cardiac arrest in special situations: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010 Nov 2;122(18 Suppl 3):S829-61. doi: 10.1161/CIRCULATIONAHA.110.971069. Erratum in: Circulation. 2011 Feb 15;123(6):e239. Erratum in: Circulation. 2011 Oct 11;124(15):e405. PMID: 20956228.
Amniotic fluid embolism (AFE) is a rare (1-2/100,000 deliveries) but frequently catastrophic obstetric emergency. Typically occurring during labor or shortly after delivery of the placenta, it is characterized by the sudden onset of cardiovascular and respiratory failure with no other identifiable cause followed by the rapid onset of disseminated intravascular coagulopathy (DIC).
Etiology and PathophysiologyTop
The exact pathogenesis of AFE remains unclear. It is hypothesized that the disruption of the maternal-fetal barrier in labor allows for the passage of amniotic fluid, fetal cells, or other fetal debris into the maternal blood circulation, which in rare cases can trigger an anaphylactoid-type reaction. The acute onset of cardiovascular collapse is thought to result from an overwhelming activation of maternal inflammatory mediators in response to the fetal material. Vasospasm of the pulmonary vasculature, hypoxia, and a rapid rise in right ventricular pressure leads to acute right heart failure and, in many cases, cardiac arrest. If the mother survives this first insult, severe dilatation of the right ventricle causes relative left ventricular compression and elevated filling pressures with decreased left ventricular volume and stroke volume. The resultant ventricular failure leads to pulmonary edema and hypotension. The subsequent onset of disseminated consumptive coagulopathy is thought to be secondary to maternal exposure to prothrombotic substances and activators in fetal fluid and injured blood vessels, often exacerbated by the sequelae of a maternal arrest (Table 15.1-1).
Several risk factors have been identified, including maternal factors (multiparity, advanced maternal age >35 years, gestational diabetes mellitus, eclampsia) and delivery-related factors (medical induction of labor, placenta abruption/previa, caesarian section, and instrumental vaginal delivery).
Clinical FeaturesTop
The most common manifestations of AFE are cardiac arrest (40% in survivors and 90% in nonsurvivors), hemorrhage (70%), coagulopathy (60%), hypotension (50%-70%), arrhythmia (25%), dyspnea (30%-45%), seizures (15%), and fetal distress (35%).
DiagnosisTop
The Society for Maternal-Fetal Medicine and the Amniotic Fluid Embolism Foundation proposed the following 4 criteria, all of which have to be met for the diagnosis of AFE to be made. The presence of any of the first 3 should raise suspicion for AFE:
1) Sudden onset of cardiac arrest, or both hypotension (systolic blood pressure <90 mm Hg) and respiratory distress (dyspnea, cyanosis, or oxygen saturation <90%).
2) Presence of DIC before loss of sufficient blood leading to dilutional or shock-related consumptive coagulopathy. DIC diagnosis during pregnancy requires ≥3 points of the following, as per the International Society on Thrombosis and Haemostasis:
a) Platelet count <100×109/L.
b) Prolonged prothrombin time (PT) or international normalized ratio (INR).
c) Fibrinogen level <200 mg/L.
3) Onset of hemodynamic instability and coagulopathy during labor or within 30 minutes of delivery of the placenta.
4) Lack of fever (<38.0 degrees Celsius) during labor.
In a critically ill pregnant patient, several diagnoses should be rapidly excluded. Use the following mnemonic BEAU-CHOPS for causes of maternal collapse:
1) Bleeding/DIC.
2) Embolism (coronary, pulmonary, amniotic fluid, venous air).
3) Anesthetic agent complications, anaphylaxis.
4) Uterine atony and hemorrhage.
5) Cardiac disease (myocardial infarction, ischemia, aortic dissection, cardiomyopathy, valvular diseases).
6) Hypertension, preeclampsia, eclampsia.
7) Other (4 H’s and T’s from advanced cardiac life support [ACLS]; see Cardiac Arrest).
8) Placenta abruption/previa.
9) Sepsis.
ManagementTop
The management, which is mostly supportive, also includes rapid exclusion of alternative etiologies for maternal cardiovascular collapse (see Differential Diagnosis, above). Optimal treatment for this rare but potentially fatal disease includes immediate consultation with a multidisciplinary team (obstetrics and gynecology, critical care, anesthesia, and maternal-fetal specialists) to increase the chances of maternal and neonatal survival with good outcomes.
In case of cardiac arrest, the first step is immediate activation of the maternal cardiac arrest code team to optimize maternal and fetal resuscitation. The goals are:
1) In case of cardiopulmonary arrest, maintenance of the airway, breathing, and circulation, including good quality cardiopulmonary resuscitation (CPR) modified for pregnant patients, as per the American Heart Association guidelines (Figure 15.1-1).
a) In an arrest situation, the patient should be placed supine for chest compressions with manual left uterine displacement (Figure 15.1-2).
b) Overall, the ACLS guidelines should be followed, with particular attention to relieving aortocaval compression.
c) Fetal delivery: In case of cardiac arrest, if the mother is still pregnant, immediate delivery (ideally within 4 minutes of onset of the ACLS protocol) is suggested, so as to aid in maternal resuscitation.
2) The remaining points apply to arrest and nonarrest situations:
a) Efforts to consider and exclude other possible/probable treatable causes and the rest of H’s and T’s from the ACLS guidelines (see Cardiac Arrest).
b) Placement of the patient in a left lateral decubitus position (>15-degree tilt) or manual left uterine displacement to relieve aortocaval compression to optimize blood return to the heart (Figure 15.1-2). These maneuvers are particularly indicated after 20 weeks’ gestation or when the uterine fundus is at or above the level of the umbilicus.
c) Control of hemorrhage and reversal of coagulopathy: Massive transfusion of blood products is usually required to achieve hemostasis and control bleeding. Institution-specific massive transfusion protocols should be followed.
d) Consideration of real-time transthoracic or transesophageal echocardiography (TEE) to quantify cardiac function.
e) Early institution of extracorporeal support to treat cardiopulmonary failure, if available.
PrognosisTop
Maternal mortality remains high, in the range of 20% to 30%, which is decreased from the previously reported mortality of 60%. Among survivors of the initial insult, especially after cardiac arrest, ~40% of patients have permanent neurologic injury. Neonatal survival depends mainly on the arrest-to-delivery time and may approach 80%.
Tables and FiguresTop
|
Trigger | |
|
Passage of amniotic fluid, fetal cells, and debris into maternal circulation |
Activation of immunologic and humoral inflammatory cascade |
|
Phase I | |
|
Vasoconstriction of pulmonary vessels |
RV dilatation as a result of increased afterload → RV dilatation → RV failure → septum deviation towards LV → decreased LV volume with increased end-diastolic pressure and decreased cardiac output → severe hypotension and hypoxemia → cardiac arrest |
|
Phase II | |
|
If patient survived initial insult |
Dilatation of right side persists → septal deviation towards LV persists → elevated filling pressures of the heart with decreased LV volume → pulmonary edema and decreased cardiac output |
|
Fetal complications |
Maternal complications |
|
– Hypoxemia – Fetal heart deceleration – Hypoxic ischemic encephalopathy |
– Hypotension and cardiac arrest – DIC and bleeding – Hypoxic ischemic encephalopathy |
|
Adapted from N Engl J Med. 2019 Oct 24;381(17):1664-1673. | |
|
DIC, disseminated intravascular coagulation; LV, left ventricle; RV, right ventricle. | |
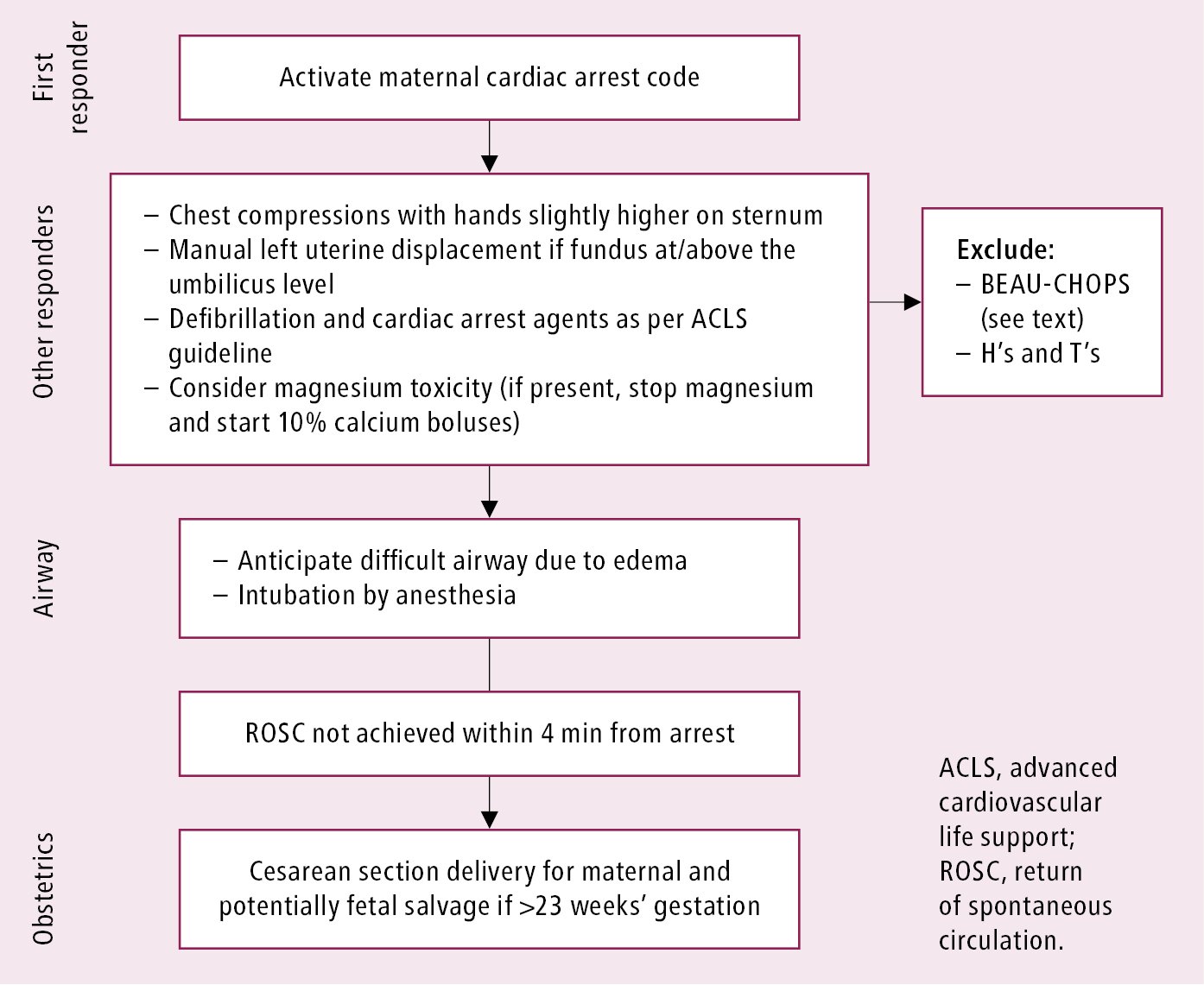
Figure 15.1-1. Maternal cardiac arrest algorithm. Adapted from Circulation. 2015 Nov 3;132(18):1747-73.
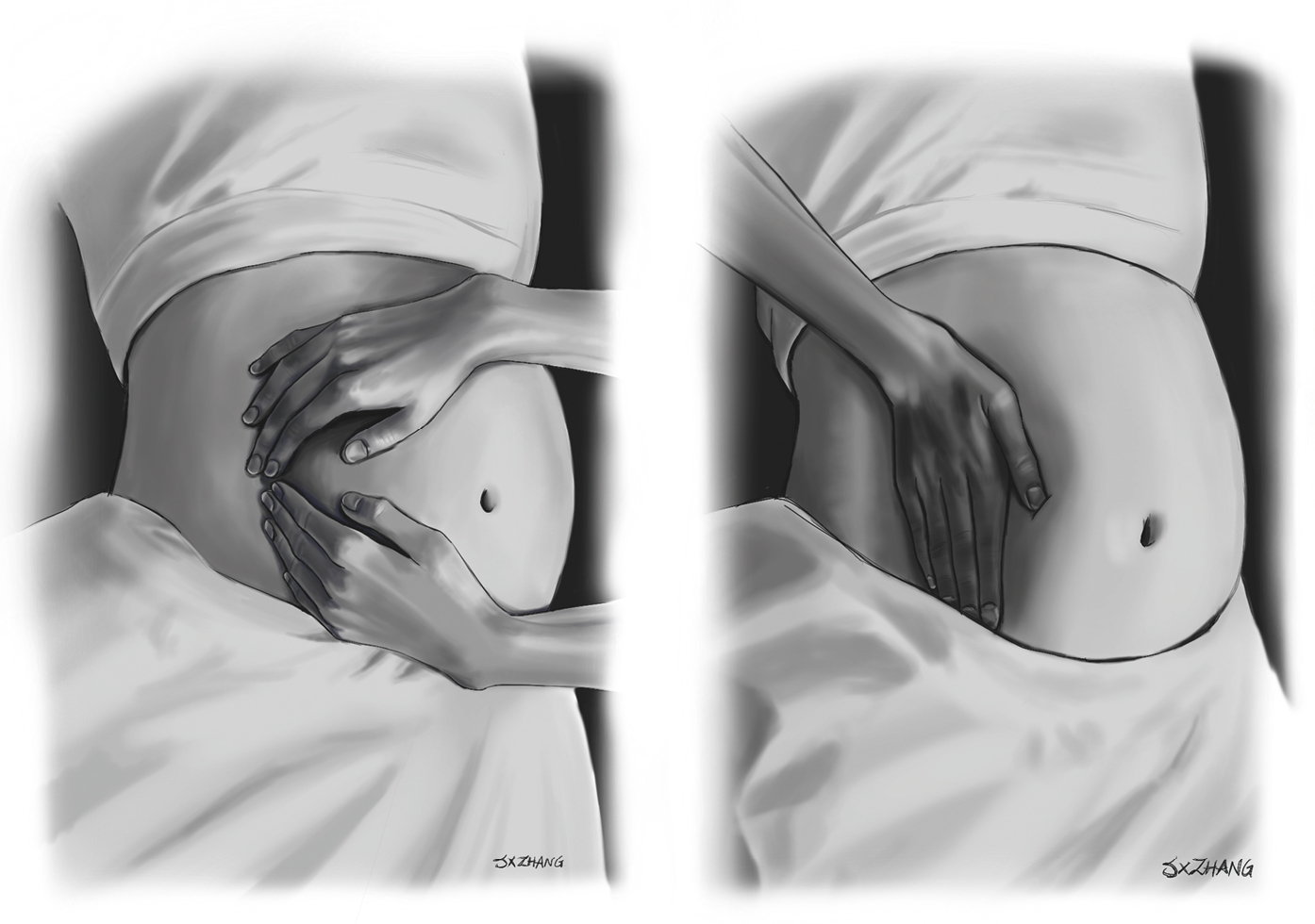
Figure 15.1-2. Manual left uterine displacement by the 2-hand and 1-hand techniques. Illustration courtesy of Dr Shannon Zhang.