Van Gelder IC, Rienstra M, Bunting KV, et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2024;45(36):3314-3414. doi:10.1093/eurheartj/ehae176
Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines [published correction appears in Circulation. 2024 Jan 2;149(1):e167. doi: 10.1161/CIR.0000000000001207.] [published correction appears in Circulation. 2024 Feb 27;149(9):e936. doi: 10.1161/CIR.0000000000001218.] [published correction appears in Circulation. 2024 Jun 11;149(24):e1413. doi: 10.1161/CIR.0000000000001263.]. Circulation. 2024;149(1):e1-e156. doi:10.1161/CIR.0000000000001193
Glikson M, Nielsen JC, Kronborg MB, et al; ESC Scientific Document Group. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. 2021 Sep 14;42(35):3427-3520. doi: 10.1093/eurheartj/ehab364. PMID: 34455430.
Andrade JG, Aguilar M, Atzema C, et al. The 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society Comprehensive Guidelines for the Management of Atrial Fibrillation. Can J Cardiol. 2020 Dec;36(12):1847-1948. doi:10.1016/j.cjca.2020.09.001. Epub 2020 Oct 22. PMID: 33191198.
Brugada J, Katritsis DG, Arbelo E, et al; ESC Scientific Document Group. 2019 ESC Guidelines for the management of patients with supraventricular tachycardia: The Task Force for the management of patients with supraventricular tachycardia of the European Society of Cardiology (ESC). Eur Heart J. 2020 Feb 1;41(5):655-720. doi: 10.1093/eurheartj/ehz467. Erratum in: Eur Heart J. 2020 Nov 21;41(44):4258. PMID: 31504425.
January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019 Jul 9;74(1):104-132. doi: 10.1016/j.jacc.2019.01.011. Epub 2019 Jan 28. Erratum in: J Am Coll Cardiol. 2019 Jul 30;74(4):599. PMID: 30703431.
Kusumoto FM, Schoenfeld MH, Barrett C, et al. 2018 ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2019 Sep;16(9):e128-e226. doi: 10.1016/j.hrthm.2018.10.037. Epub 2018 Nov 6. PMID: 30412778.
January CT, Wann LS, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014 Dec 2;130(23):e199-267. doi: 10.1161/CIR.0000000000000041. Epub 2014 Mar 28. Erratum in: Circulation. 2014 Dec 2;130(23):e272-4. PMID: 24682347; PMCID: PMC4676081.
Priori SG, Wilde AA, Horie M, et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013 Dec;10(12):1932-63. doi: 10.1016/j.hrthm.2013.05.014. Epub 2013 Aug 30. Review. PMID: 24011539.
Definition, Etiology, PathogenesisTop
Atrial fibrillation (AF) is the most common supraventricular tachyarrhythmia (prevalence in adults, 1%-2%). It is characterized by fast (350-700 beats/min) and irregular atrial activation, which results in the loss of hemodynamic effectiveness of atrial contractions and a concomitant irregular ventricular rhythm. Ventricular rate depends on the electrophysiologic features of the atrioventricular (AV) node, autonomic function, and effects of drugs. It may be normal (resting heart rates, 60-100 beats/min), fast (tachyarrhythmia), or slow (bradyarrhythmia).
AF classification: Figure 3.4-1. In patients with episodes of AF that may be classified as different categories, consider the predominant type of AF.
Risk factors: The main risk factors for developing AF include hypertension, overweight, untreated sleep apnea, and alcohol intake. A variety of other cardiac and extracardiac conditions can also increase the risk of developing AF, including valvular heart disease (primarily mitral valve disease), heart failure irrespective of its cause, cardiomyopathies, diabetes (and other cardiac risk factors), thyrotoxicosis, pulmonary diseases, and chronic kidney disease. However, AF can also be observed in individuals without structural heart disease.
Clinical Features and Natural HistoryTop
Symptoms may include palpitations, weakness, poor exercise tolerance, syncope, or dizziness. Patients may also be asymptomatic.
The European Heart Rhythm Association (EHRA) score used for the classification of AF-related symptoms:
1) EHRA class I: Asymptomatic.
2) EHRA class II: Mild symptoms not affecting normal daily activity.
3) EHRA class III: Severe symptoms affecting daily activity.
4) EHRA class IV: Disabling symptoms.
Signs include grossly irregular heart rate, irregular pulse, and pulse deficit (not every ventricular contraction, especially if occurring shortly after the previous one, may be sufficiently strong to transmit an arterial pulse wave through the peripheral artery).
A detailed history and analysis of the available medical records are important. Paroxysmal AF is self-limiting and resolves within 7 days. Persistent AF does not resolve spontaneously, lasts >7 days, or both. Long-standing persistent AF refers to uninterrupted AF lasting >1 year. Permanent AF refers to situations where efforts to restore sinus rhythm have either failed or been forgone. These categories are not mutually exclusive and it is common for patients with one type of AF to exhibit overlapping features of another type.
DiagnosisTop
1. Electrocardiography (ECG): A grossly irregular rhythm, P waves absent or not clearly identifiable, fibrillatory waves (Figure 3.4-2).
2. Holter ECG monitoring: Long-term monitoring (24 hours to 14 days) may be useful in the identification of episodes of paroxysmal AF.
3. Echocardiography: This is useful in the identification of structural heart disease and assessing left ventricular function and left atrium size. The larger the left atrium, the less likely it is to achieve a stable sinus rhythm.
4. New technologies can also aid in the diagnosis of AF (watches with optical sensors, mobile cardiac monitoring devices).
Supraventricular tachycardia: see Figure 3.4-2.
TreatmentTop
Classification of antiarrhythmic drugs: see Table 3.4-1.
Antiarrhythmic agents: see Table 3.4-2.
The management of paroxysmal AF depends on episode duration, symptoms, and hemodynamic status:
1) AF <48 hours:
a) In patients with symptomatic AF lasting <48 hours, an acute rhythm control strategy should be favored in order to avoid left atrial remodeling due to persistent AF.
b) If a rhythm control strategy is chosen, cardioversion may be achieved either pharmacologically or electrically. In patients with no structural heart disease and no contraindications, consider oral flecainide 300 mg plus an oral dose of an AV nodal blocker, like a beta-blocker, diltiazem, or verapamil; or propafenone 600 mg as a single dose (no need to add an AV nodal blocker). In all other cases consider IV amiodarone but remember that conversion to sinus rhythm usually takes longer (8-12 hours).Evidence 1Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due to heterogeneity of the studied populations. Cordina J, Mead G. Pharmacologic cardioversion for atrial fibrillation and flutter. Cochrane Database Syst Rev. 2005 Apr 18;(2):CD003713. Review. Update in: Cochrane Database Syst Rev. 2017 Nov 15;11:CD003713. PubMed PMID: 15846675. Mead GE, Elder AT, Flapan AD, Kelman A. Electrical cardioversion for atrial fibrillation and flutter. Cochrane Database Syst Rev. 2005 Jul 20;(3):CD002903. Review. Update in: Cochrane Database Syst Rev. 2017 Nov 15;11:CD002903. PubMed PMID: 16034878. In patients with significant hemodynamic disturbances or severe chest pain caused by rapid AF, perform urgent electrical cardioversion.
c) Elderly patients with comorbidities and mild symptoms may benefit from a rate control strategy. For rate control, consider beta-blockers, diltiazem, or verapamil. Digoxin is usually not considered in monotherapy but combined with any of the other medications.
2) AF >48 hours:
a) When considering either electrical or pharmacologic cardioversion of AF, patients should receive adequate anticoagulation treatment to prevent thromboembolic complications. The risk of thromboembolism is the same whether conversion is achieved electrically or pharmacologically (see Complications, below).
b) In case of an AF episode lasting >48 hours, as an alternative to adequate anticoagulation (duration ≥3 weeks), transesophageal echocardiography (TEE) may be performed to exclude a left atrial appendage thrombus, which would be a contraindication to cardioversion.
1. Paroxysmal AF:
1) Eliminate risk factors that may have triggered the arrhythmia, such as alcohol, sleep apnea, or uncontrolled hypertension.
2) If episodes are infrequent and short-lasting, follow up with no antiarrhythmics or consider rate-control medications. If episodes are more frequent but still <1 episode per week, patients with no structural heart disease may be treated with the “pill-in-the-pocket” approach: a single dose of propafenone 600 mg (450 mg in patients <70 kg) or flecainide 300 mg plus an AV nodal blocker (eg, metoprolol, bisoprolol, diltiazem, or verapamil),Evidence 2Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). Low Quality of Evidence (low confidence that we know true effects of the intervention). Quality of Evidence lowered due to the observational nature of data and indirectness of the population and outcomes measured. Saborido CM, Hockenhull J, Bagust A, Boland A, Dickson R, Todd D. Systematic review and cost-effectiveness evaluation of 'pill-in-the-pocket' strategy for paroxysmal atrial fibrillation compared to episodic in-hospital treatment or continuous antiarrhythmic drug therapy. Health Technol Assess. 2010 Jun;14(31):iii-iv, 1-75. doi: 10.3310/hta14310. Review. PubMed PMID: 20569652. provided the efficacy and safety of the drug have been previously confirmed in a hospital setting. There is no need to add a nodal blocker on top of propafenone.
3) Patients with frequent episodes should receive daily medications according to their profile. In young patients with no heart disease, flecainide or propafenone is preferred for a rhythm control strategy. In patients with coronary artery disease, amiodarone or dronedarone are options and sotalol may be considered. In patients with left ventricular dysfunction or contraindications for other antiarrhythmics, amiodarone should be considered.
2. In patients with persistent AF, choose a management strategy:
1) Rhythm-control strategy: Restore sinus rhythm either electrically or pharmacologically and attempt to maintain sinus rhythm with antiarrhythmic agents, ablation, or both.
2) Rate-control strategy: Do not attempt to restore sinus rhythm but ensure optimal control of the ventricular rate during AF using an exercise test or Holter monitor to maintain an average 24-hour heart rate of <110 beats/min and no symptoms. Rate control can be achieved with one or more of beta-blockers, diltiazem, verapamil, and digoxin, or with ablation of the AV node (and pacemaker implantation).
3) Asymptomatic patients with persistent AF: Recent studies (EAST-AFNET 4 trial) have revealed that rhythm control is associated with a lower risk of adverse cardiovascular outcomes than usual care among patients with recently (within 1 year) diagnosed AF.Evidence 3Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due to indirectness. Kirchhof P, Camm AJ, Goette A, et al; EAST-AFNET 4 Trial Investigators. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. N Engl J Med. 2020 Oct 1;383(14):1305-1316. doi: 10.1056/NEJMoa2019422. Epub 2020 Aug 29. PMID: 32865375. If the left atrium is severely dilated, rhythm control is less likely to succeed.
3. Permanent AF: The goal of treatment is ventricular rate control. The target is to maintain a heart rate <110 beats/min. If left ventricular dysfunction is present, the target for rate control should be more stringent.
Drug selection: Table 3.4-1.
1. Catheter ablation: Most AF episodes are thought to originate in the pulmonary veins. The current method for AF ablation focuses on the isolation of the pulmonary veins using radiofrequency, cryoablation, or pulsed field ablation. It is recommended in patients with symptomatic paroxysmal AF in whom treatment with ≥1 class I or class III antiarrhythmic agents has been unsuccessful and in patients who prefer to avoid medication. Catheter ablation has shown superior efficacy in maintaining sinus rhythm when compared with antiarrhythmic drugs (the success rate for maintaining sinus rhythm at 1 year with no episodes of AF is ~70%).
2. Surgical ablation: An option in patients undergoing cardiac surgery, commonly with mitral valve disease, who have persistent AF. Surgical ablation has shown better results than catheter ablation for persistent AF with the added benefit of left atrial appendage removal, a procedure that has shown a decrease in the risk of stroke or systemic embolism.
3. Catheter ablation of the AV junction with pacemaker implantation: This may be considered as a resource in patients with paroxysmal or permanent AF who remain symptomatic despite other treatment strategies. This is a final rate-control strategy.
ComplicationsTop
The most significant complications of AF include thromboembolic events, mainly ischemic stroke. They are associated with the formation of a thrombus in the left atrium (most commonly in the left atrial appendage).
1. Long-term prevention of thromboembolism: In every patient with AF assess the risk of thromboembolic complications using the CHA2DS2-VASc score (congestive heart failure, hypertension, age ≥75 years [doubled], diabetes, stroke/transient ischemic attack/thromboembolism [doubled], vascular disease [prior myocardial infarction, peripheral artery disease, aortic plaque], age 65-74 years, sex category [female]). Long-term anticoagulation is recommended in patients with a CHA2DS2-VASc score ≥1 except if the single risk factor is female sex (Table 3.4-2) or if there is a high risk of intracranial bleeding (Table 3.4-3). Recommended prophylaxis: Figure 3.4-3.
2. Prophylactic antithrombotic treatment in patients treated with cardioversion:
1) Patients with AF lasting ≥48 hours or of unknown duration: Before attempting to restore sinus rhythm (electrical or pharmacologic cardioversion), use a vitamin K antagonist (VKA) for ≥3 weeks before cardioversion and 4 weeks after the procedure (maintain an international normalized ratio [INR] of 2.0-3.0; note that in some patients longer anticoagulation may be indicated; consider risk factors for thromboembolic complications). Dabigatran, rivaroxaban, or apixaban may be used instead of VKAs. If a left atrial appendage thrombus has been excluded with TEE, it would be safe to proceed with cardioversion before completing 3 weeks of anticoagulation. In any case, 4 weeks of anticoagulation are needed after cardioversion.
If urgent cardioversion is required in patients with AF lasting ≥48 hours or of unknown duration, exclude the presence of a thrombus using TEE, if available. Before cardioversion administer IV unfractionated heparin (or subcutaneous low-molecular-weight heparin). After cardioversion use an oral anticoagulant for ≥4 weeks, depending on the risk factors for thromboembolism (CHA2DS2-VASc score; see above).
2) In patients with AF of documented duration <48 hours, pharmacologic or electrical cardioversion may be performed immediately after the administration of heparin. Oral anticoagulation should be started immediately for ≥4 weeks, depending on the risk factors for thromboembolism (CHA2DS2-VASc score; see above).
3. Prevention of thromboembolism in patients with AF after percutaneous coronary intervention (PCI): This field is rapidly changing and no uniform strong recommendations are relevant in all patients. More recently, dual therapy with modified direct oral anticoagulant (DOAC) doses (in the range of 25%-75% of the full anticoagulation dose) plus clopidogrel have been reported to be noninferior to triple therapy with a VKA with respect to the risk of thromboembolic events, with fewer bleeding events than in the case of triple therapy. In patients after PCI in the context of an acute coronary syndrome, the benefit of triple therapy seems limited to the first 30 days, and then acetylsalicylic acid can be safely discontinued while maintaining clopidogrel plus a DOAC. Prasugrel and ticagrelor should be avoided in conjunction with DOACs.
Tables and FiguresTop
|
Concomitant cardiac disease |
Treatment methoda |
|
No significant structural heart disease |
1) Pharmacotherapy: – Recommended: dronedarone, flecainide, propafenone – May be considered: sotalolb 2) Percutaneous ablationc |
|
CAD, significant valvular heart disease, HFpEF |
1) Pharmacotherapy: – Recommended: amiodarone, dronedarone – May be considered: sotalolb 2) Percutaneous ablationc |
|
HFrEF |
1) Pharmacotherapy: amiodarone 2) Percutaneous ablationc,d |
|
a The choice between pharmacotherapy and percutaneous ablation depends on the type of treatment (first line vs second line), type of AF (paroxysmal vs persistent), and the patient’s decision. b Patients treated with sotalol should be monitored for proarrhythmia (assess for QT prolongation with periodical ECG; our pattern is baseline 1 week after starting treatment and every 6 months afterwards). c Pulmonary vein isolation with radiofrequency or cryoballoon ablation. d Ablation as first-line treatment is usually reserved for patients with HF secondary to tachycardia-induced cardiomyopathy. |
|
|
Adapted from the 2020 European Society of Cardiology guidelines. |
|
|
AF, atrial fibrillation; CAD, coronary artery disease; ECG, electrocardiography; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction. |
|
|
Risk factor |
Score |
|
|
CHADS2 |
CHA2DS2-VASc |
|
|
Congestive heart failure or LV dysfunctiona |
1 |
1 |
|
Hypertension |
1 |
1 |
|
Age ≥75 years |
1 |
2 |
|
Diabetes mellitus |
1 |
1 |
|
History of stroke, TIA, or thromboembolisma |
2 |
2 |
|
Vascular diseaseb |
– |
1 |
|
Age 65-74 years |
– |
1 |
|
Female sex |
– |
1 |
|
a Left ventricular dysfunction and other thromboembolic event are included only in the CHA2DS2-VASc score. b History of myocardial infarction, peripheral artery disease, or atherosclerotic plaque in aorta. |
||
|
LV, left ventricle; TIA, transient ischemic attack. |
||
|
Clinical characteristic |
Points |
|
|
Hypertensionb |
1 |
|
|
Abnormal kidneyc and liver functiond |
1 or 2 |
|
|
Stroke |
1 |
|
|
Bleedinge |
1 |
|
|
Labile INRsf |
1 |
|
|
Elderly (age >65 years) |
1 |
|
|
Drugsg or alcohol |
1 or 2 |
|
|
Interpretation: Score ≥3 points: High risk |
||
|
a Severe bleeding, that is, intracranial, requiring hospitalization, associated with >2 g/dL decrease in hemoglobin levels, or requiring transfusion. b Systolic blood pressure >160 mm Hg. c Chronic dialysis or kidney transplant or serum creatinine >200 micromol/L. d Chronic liver disease (eg, cirrhosis) or biochemical evidence of significant liver derangement (eg, bilirubin >2 × ULN + ALT/AST/alkaline phosphatase >3 × ULN). e History of bleeding, predisposition to bleeding, or both (eg, bleeding diathesis, anemia). f Unstable/high INR or INR frequently (eg, >40% of test results) outside the therapeutic range. g Concomitant treatment with antiplatelet agents or NSAIDs. |
||
|
ALT, alanine aminotransferase; AST, aspartate aminotransferase; INR, international normalized ratio; NSAID, nonsteroidal anti-inflammatory drug; ULN, upper limit of normal. |
||
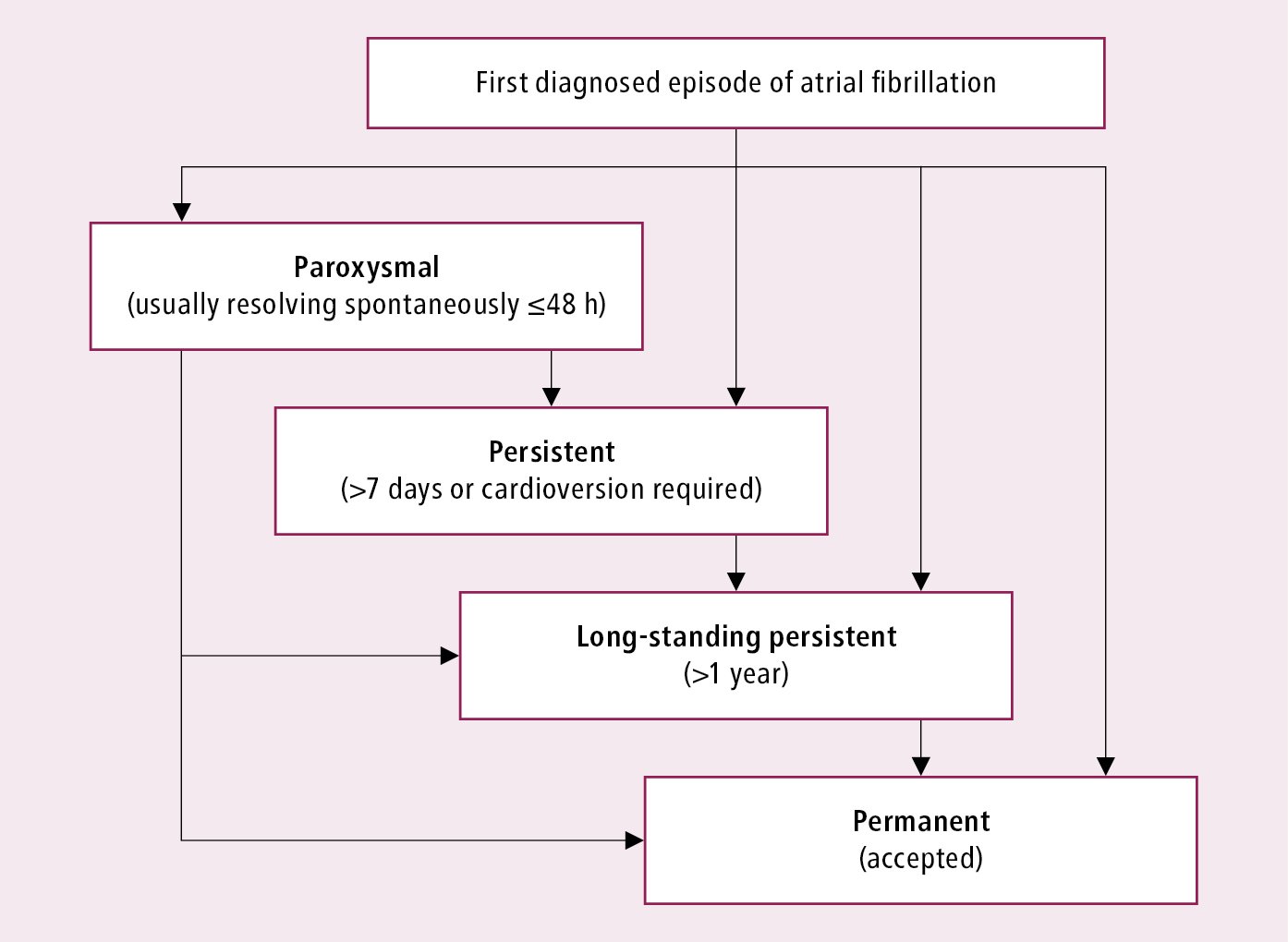
Figure 3.4-1. Classification of atrial fibrillation. Based on the European Society of Cardiology guidelines.
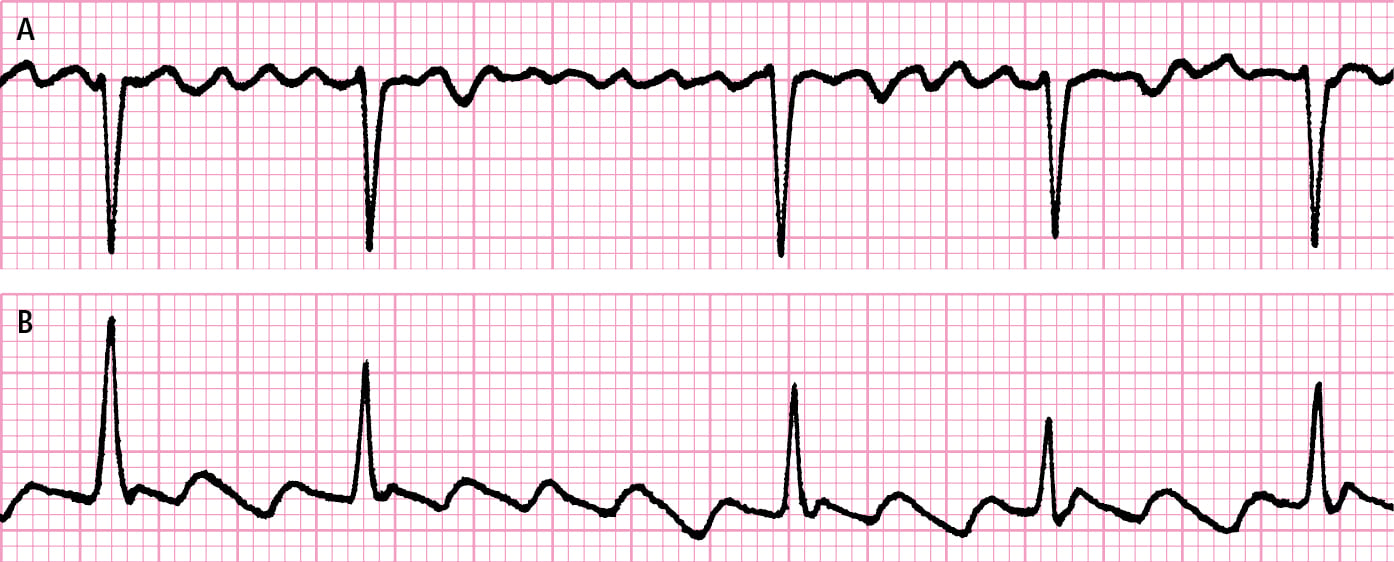
Figure 3.4-2. Atrial fibrillation and atrial flutter. A, polymorphic F waves replacing P waves during atrial fibrillation. B, monomorphic biphasic F waves replacing P waves during atrial flutter.
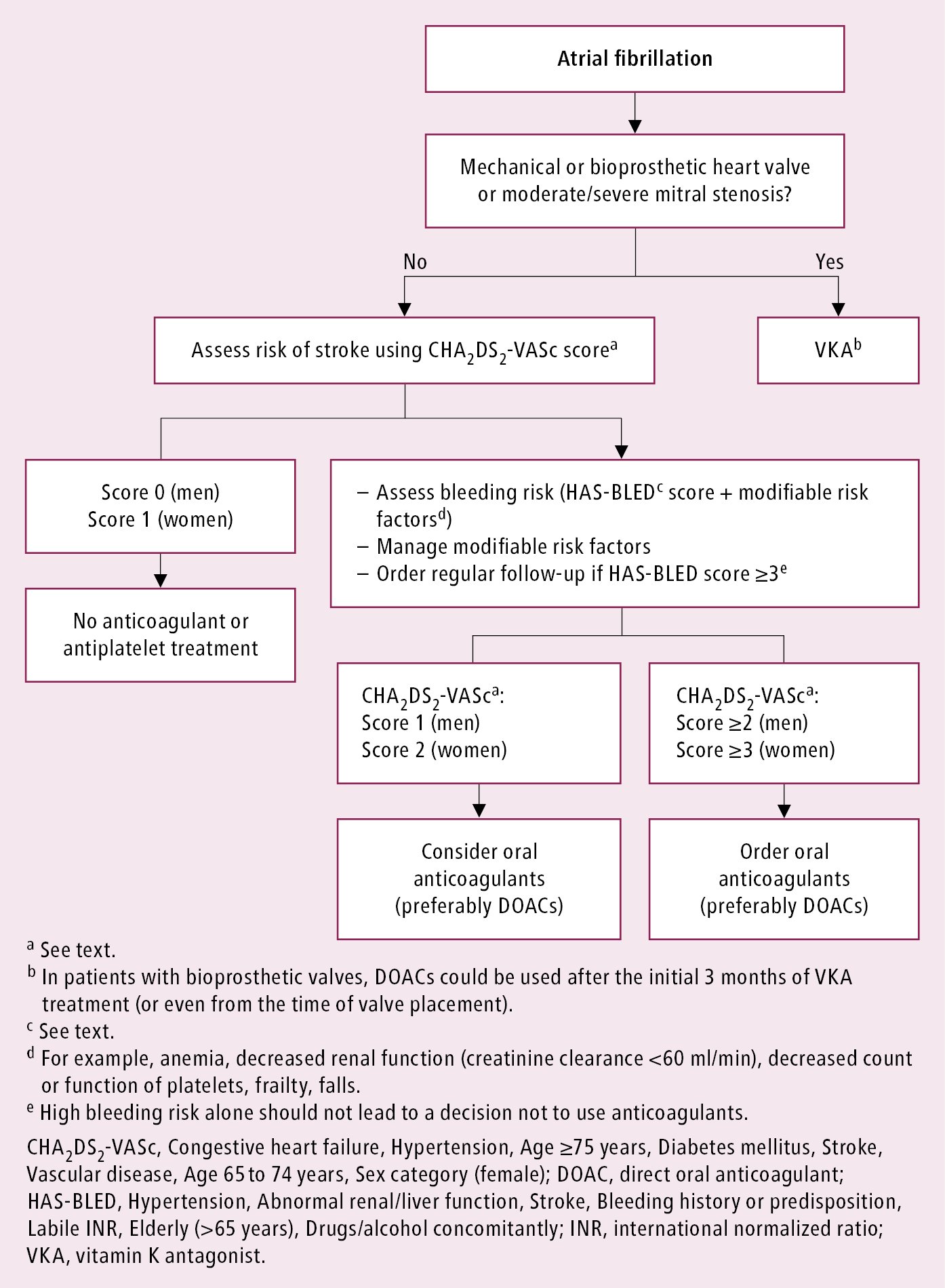
Figure 3.4-3. Algorithm for prevention of thromboembolism in patients with atrial fibrillation. Adapted from the 2020 European Society of Cardiology guidelines.