Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid. 2017 Mar;27(3):315-389. doi: 10.1089/thy.2016.0457. Erratum in: Thyroid. 2017 Sep;27(9):1212. PMID: 28056690.
Ross DS, Burch HB, Cooper DS, et al. 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid. 2016 Oct;26(10):1343-1421. Erratum in: Thyroid. 2017 Nov;27(11):1462. PMID: 27521067.
Bartalena L, Baldeschi L, Boboridis K, et al; European Group on Graves' Orbitopathy (EUGOGO). The 2016 European Thyroid Association/European Group on Graves' Orbitopathy Guidelines for the Management of Graves' Orbitopathy. Eur Thyroid J. 2016 Mar;5(1):9-26. doi: 10.1159/000443828. Epub 2016 Mar 2. PubMed PMID: 27099835; PMCID: PMC4836120.
Definition, Etiology, PathogenesisTop
Subclinical hyperthyroidism/thyrotoxicosis is defined biochemically as the presence of low thyroid-stimulating hormone (TSH) and normal free thyroxine (FT4) and free triiodothyronine (FT3) concentrations. Patients can be asymptomatic or symptoms of thyrotoxicosis may be present.
Overt primary thyrotoxicosis refers to suppressed TSH with high levels of FT4, FT3, or both. Patients can be asymptomatic or more commonly present with symptoms of hyperthyroidism.
Thyrotoxicosis is a clinical state that results from any condition leading to high thyroid hormone action in tissues.
Hyperthyroidism is a form of thyrotoxicosis caused by high synthesis and secretion of thyroid hormone by the thyroid gland.
Thyrotoxic crisis (thyroid storm) is a life-threatening, acute, and rapid collapse of homeostasis, developing as a result of undiagnosed or inadequately treated thyrotoxicosis and involving altered mental status that may progress to coma, cardiac and multiorgan failure, shock, and death.
Causes of the excess of thyroid hormones: see Figure 1.
Clinical Features and Natural HistoryTop
Thyrotoxicosis usually develops over the course of several months. It may also appear suddenly (eg, when associated with exposure to iodinated contrast media), develop over several years (autonomously functioning nodule, toxic multinodular goiter), or be transient and resolve spontaneously (subacute or postpartum thyroiditis). Various causes of thyrotoxicosis coexist rarely; for instance, a hyperfunctioning thyroid nodule in a patient with Graves disease (GD) may produce an unusual clinical course without periods of remission typical of GD.
Subclinical Thyrotoxicosis/Hyperthyroidism
Subclinical thyrotoxicosis is defined biochemically as the presence of low TSH and normal FT4 and FT3 levels. In ~50% of cases serum TSH levels spontaneously normalize, and the estimated risk of progression to overt thyrotoxicosis is ~5% per year (the progression may be triggered by exposure to iodine). Patients with subclinical thyrotoxicosis may be asymptomatic or have associated symptoms of thyrotoxicosis, including tachycardia, supraventricular arrhythmias (atrial fibrillation, premature supraventricular contraction), and (rarely) ventricular arrhythmias. If long-standing and untreated, subclinical thyrotoxicosis leads to decreased bone mineral density, and in elderly patients with TSH levels <0.1 mIU/L, it may be associated with elevated risk of cardiovascular complications and increased mortality.
1. General symptoms: Weight loss despite normal or increased appetite (elderly patients may present with weight loss and less of an increase in appetite), weakness, and heat intolerance.
2. Neuropsychiatric symptoms: Anxiety, irritability, emotional lability, hyperactivity (hyperkinetic behavior), impaired concentration, insomnia; rarely psychotic symptoms (usually in those with preexisting psychiatric disorders), fine tremor, and hyperreflexia.
3. Ocular symptoms:
1) Eyelid retraction and stare can be seen in any form of thyrotoxicosis (resulting from sympathetic overactivity).
2) Ocular symptoms resulting from myopathy accompanying GD (Graves orbitopathy [GO]; see Graves Disease): Periorbital edema, congestion or redness of the conjunctiva and swelling of the conjunctiva (chemosis), proptosis, upward gaze limitation, corneal involvement (due to the inability to close the eyes completely), and optical nerve involvement in severe cases.
4. Cutaneous symptoms: Excessive sweating and skin hyperemia (warm, pink, moist, and excessively smooth skin); rarely hyperpigmentation (not including mucosal surfaces) or urticaria; hair loss, thinning, and breaking; thin and fragile nails, which may separate from nail beds (onycholysis); thyroid dermopathy (pretibial myxedema) (Figure 2) and acropachy may be present in GD.
5. Musculoskeletal symptoms: Decrease of muscle mass and strength (in more severe thyrotoxicosis); in severe cases, thyrotoxic myopathy involving muscles of the face and distal limbs. Involvement of extraocular muscles may then mimic myasthenia gravis.
6. Abnormalities of the neck: In some patients increased neck circumference and sensation of pressure are observed. On physical examination the thyroid gland is of normal size or enlarged (GD, nodular goiter, thyroiditis); there may be ≥1 nodule or tenderness (painful thyroiditis). Finding a bruit on physical examination of the thyroid gland is indicative of a vascular goiter (typical of GD) and if ≥1 nodule is present, it is necessary to consider toxic nodular goiter in the differential diagnosis (the presence of nodules does not exclude GD).
7. Respiratory symptoms: Dyspnea caused by respiratory muscle weakness, increased oxygen consumption, and rarely by tracheal compression due to an enlarged thyroid gland.
8. Cardiovascular symptoms: Palpitations, hyperkinetic circulation (tachycardia, systolic hypertension, widened pulse pressure, loud heart sounds); arrhythmia with premature ventricular contractions or atrial fibrillation, systolic murmurs (due to mitral prolapse or regurgitation), sometimes end-diastolic murmurs; symptoms of heart failure, particularly in patients with preexisting heart disease.
9. Gastrointestinal symptoms: Hyperdefecation (increased stool frequency without increase in overall volume) is common. In severe thyrotoxicosis hepatomegaly and jaundice may develop due to liver damage.
10. Abnormalities of the reproductive system: Decreased libido, oligomenorrhea (generally with ovulation), amenorrhea, erectile dysfunction, or gynecomastia.
Thyrotoxic Crisis (Thyroid Storm)
Thyrotoxic crisis is an acute life-threatening exacerbation of thyrotoxicosis in patients who have undiagnosed or inadequately treated thyrotoxicosis and superimposed precipitant factors (eg, surgery, infection, trauma, iodinated contrast media exposure). In case of a sudden deterioration in the status of a patient with hyperthyroidism, always consider the possibility of impending or overt thyrotoxic crisis. The predominant signs and symptoms may be those related to the underlying disease that precipitated thyrotoxic crisis.
1. Precedent symptoms: Agitation, insomnia (hallucinations and other psychotic symptoms may occur), significant weight loss, worsening of tremor, fever, nausea, and vomiting.
2. Overt thyrotoxic crisis: Hyperthermia; extreme agitation and worsening of psychotic symptoms; sometimes somnolence, apathy, or even coma; in some cases status epilepticus; sudden worsening of cardiovascular symptoms (severe tachycardia, atrial fibrillation, myocardial ischemia, symptoms of heart failure, sometimes shock) and gastrointestinal symptoms of thyrotoxicosis (nausea, diarrhea, and hepatic dysfunction); signs and symptoms of dehydration (often following a period of hyperhidrosis).
DiagnosisTop
Always inquire about a family history of thyroid disorders, exposure to high doses of iodine (certain disinfecting agents [eg, iodine tincture] or expectorants; amiodarone; iodinated contrast media), and previous treatment of thyroid diseases and autoimmune diseases of other organs. Simultaneously assess thyroid function and morphology and aim at identifying the causative factors. The presence of a bruit (vascular goiter) and signs of orbitopathy indicate GD. Always make sure that serum TSH and free thyroid hormone levels are consistent and correspond with the presenting signs and symptoms.
In patients with suspected thyrotoxicosis evaluate serum TSH levels and, if abnormal, FT4 and FT3 levels and examine the thyroid gland for enlargement and presence of nodules in the parenchyma (palpable nodules or nodules detected on ultrasonography may fulfill the criteria for fine-needle biopsy [FNB]; see Goiter, Nontoxic Multinodular). TSH receptor antibodies (TRAb) allow for a reliable differential diagnosis of autoimmune (GD) and nonautoimmune causes of thyrotoxicosis.
1. Hormone tests:
1) Serum TSH concentrations are the most sensitive markers of activity of thyroid hormones. They are decreased in primary thyrotoxicosis (both overt and subclinical) and increased in secondary thyrotoxicosis (very rare TSH-producing pituitary adenoma).
2) Serum FT4 and FT3 concentrations are increased in overt thyrotoxicosis (more commonly FT4 or both FT4 and FT3 are affected; isolated FT3 elevation is rare but confirms thyrotoxicosis if TSH level is low and FT4 level is normal) and normal (often close to the upper limit of normal [ULN]) in subclinical thyrotoxicosis.
2. Other laboratory tests:
1) TRAb: A positive TRAb result confirms the diagnosis of GD.Evidence 1 Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). Low Quality of Evidence (low confidence that we know true effects of the intervention). Quality of Evidence lowered due to estimates of accuracy derived from studies at high risk of bias (selection bias and lack of blinding). Pedersen IB, Knudsen N, Perrild H, Ovesen L, Laurberg P. TSH-receptor antibody measurement for differentiation of hyperthyroidism into Graves' disease and multinodular toxic goitre: a comparison of two competitive binding assays. Clin Endocrinol (Oxf). 2001 Sep;55(3):381-90. PubMed PMID: 11589682. TRAb is especially important if molecular imaging (radioactive iodine [RAI] uptake and scan [RAIU/Scan]) is not available or if the patient has recently received iodine load (computed tomography [CT], angiography) and RAIU may give false-negative results, and in diagnosing thyroid orbitopathy (including thyroid eye disease also in patients without GD who have underlying thyroid autoimmunity). TRAb may also add to the prediction of remission in response to medical treatment and can be useful to diagnose GD in pregnancy—as then RAIU/Scan is contraindicated—and to determine the risk of GD in the fetus if the mother had prior radioactive ablation therapy or thyroidectomy.
2) Serum antibodies to thyroperoxidase (TPOAb) and antibodies to thyroglobulin (TgAb) (least specific): Measurement of TPOAb and TgAb is not suggested for the workup of patients with low TSH values,Evidence 2 Weak recommendation (downsides likely outweigh benefits, but the balance is close or uncertain; an alternative course of action may be better for some patients). Low Quality of Evidence (low confidence that we know true effects of the intervention). Quality of Evidence lowered due to the unclear accuracy of those tests to indicate the etiology of low thyroid-stimulating hormone. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002 Feb;87(2):489-99. PubMed PMID: 11836274. as they do not definitely indicate etiology (may also be present in healthy individuals and patients with nonautoimmune thyroid diseases, most frequently in subacute thyroiditis) and can lead to unnecessary tests.
3) Serum thyroglobulin Tg levels are useful only for excluding thyrotoxicosis caused by exogenous thyroid hormone excess, in which case serum Tg is low.
3. Molecular and other imaging studies:
1) RAIU measures the percentage of function of the thyroid gland. A high (typical of GD or toxic nodule or nodules) or normal uptake indicates de novo synthesis of thyroid hormone, while a low uptake suggests inflammation or destruction of the thyroid gland (such as in thyroiditis) or can be found in patients with factitious thyrotoxicosis, overreplacement with exogenous thyroid hormone, or recent iodine or amiodarone ingestion or administration.
2) Thyroid scan (scintigraphy), usually utilizing technetium pertechnetate, provides information on the radioactive molecule accumulation pattern and anatomy. Intense and homogeneous concentration of the tracer is typical of GD, while ≥1 area of increased tracer activity with a reduction or suppression of the rest of the gland indicate toxic nodular goiter. To prepare a patient for molecular imaging, discontinue all antithyroid drugs ≥5 days before the study. Inquire about any iodine-containing drugs or recent exposure to contrast media, as they reduce iodine uptake by the thyroid gland. Radionuclide tests are contraindicated in pregnancy and during breastfeeding.
3) Ultrasonography of the thyroid gland with assessment of thyroid blood flow might be an alternative diagnostic approach if available. Ultrasonography is also useful to characterize palpable nodules or hypofunctioning areas found on thyroid scintigraphy.
4) Orbital CT or magnetic resonance imaging (MRI) may be used in patients with GO to diagnose involvement of extraocular muscles if the cause of orbitopathy is unclear.
4. Cytology is used to classify thyroid nodules as malignant (thyroid cancer), suspicious, or nonmalignant in order to determine indications for surgery. For hyperfunctioning nodules observed in scintigraphy, FNB is not suggestedEvidence 3 Weak recommendation (downsides likely outweigh benefits, but the balance is close or uncertain; an alternative course of action may be better for some patients). Low Quality of Evidence (low confidence that we know true effects of the intervention). Quality of Evidence lowered due to the high risk of bias (indirectness and lack of control). Michigishi T, Mizukami Y, Shuke N, et al. An autonomously functioning thyroid carcinoma associated with euthyroid Graves' disease. J Nucl Med. 1992 Nov;33(11):2024-6. PubMed PMID: 1432166. due to the low risk of malignancy and increased risk of indeterminate results, unlike in cold (hypofunctioning) nodules.
1. Subclinical thyrotoxicosis is diagnosed on the basis of biochemical findings: Low serum TSH concentrations are accompanied by normal (sometimes close to the ULN) serum concentrations of free thyroid hormones in patients in whom other causes of low serum TSH levels (eg, treatment with glucocorticoids or dopamine, first trimester of pregnancy) have been excluded. If TSH levels are persistently low, differential diagnosis must be performed to determine the cause of excessive thyroid hormone levels (serum TRAb test and thyroid radionuclide scintigraphy).
2. Overt thyrotoxicosis:
1) Primary: Low serum TSH concentrations and elevated (above the ULN) serum concentrations of free thyroid hormones (FT4; FT4 and FT3; or rarely FT3 alone) accompanied by typical clinical manifestations or atypical signs and symptoms (thyrotoxicosis presenting mainly with atrial fibrillation, symptomatic coronary heart disease, or heart failure; very rarely apathetic thyrotoxicosis in the elderly, with dominant fatigue, apathy, depression, or even confusion).
2) Secondary or central: Elevated serum FT4 and FT3 concentrations with normal or elevated serum TSH levels.
3. Thyrotoxic crisis (thyroid storm) may occur in a person with unrecognized or inadequately treated hyperthyroidism or thyrotoxicosis in the presence of triggering factors. It should be suspected in every case of sudden deterioration in a patient with thyrotoxicosis. Multiorgan failure may develop. Assess the risk of thyrotoxic crisis using the Burch-Wartofsky criteria (Table 1).
Thyroid function abnormalities are differentiated on the basis of serum TSH, FT4, and FT3 levels and other test results (Table 2). Note other causes of thyrotoxicosis: hashitoxicosis, subacute thyroiditis or postpartum thyroiditis, trophoblastic disease, iodine-induced (including iodine in amiodarone) hyperthyroidism (see Chronic Thyroiditis, Other Types), drug-induced destruction of thyroid parenchyma (interferon alpha, interleukin 2, lithium, tyrosine kinase inhibitors, immunotherapy), excessive levothyroxine (L-T4) replacement dose, functioning follicular carcinoma metastases, struma ovarii. Consider the amiodarone-related direct toxic effect on thyroid cells (additional information on amiodarone-related conditions: Table 3). In the differential diagnosis of secondary hyperthyroidism, consider TSH-secreting pituitary adenoma and other conditions associated with elevated serum FT4 levels without TSH suppression: high T4 syndrome and thyroid hormone resistance syndrome.
TreatmentTop
General Principles of Thyrotoxicosis Treatment
The choice of treatment depends on the causes of thyrotoxicosis (see Graves Disease; see Goiter, Nontoxic Multinodular; see Toxic Thyroid Nodule), its course, and the patient’s decisions.
In persistent subclinical thyrotoxicosis with serum TSH levels <0.1 mIU/L, treatment is indicated in patients >65 years of age and in younger individuals with coexisting comorbidities (cardiovascular disease, osteoporosis, postmenopause), symptoms of thyrotoxicosis, or both; in the remaining cases, watchful waiting is acceptable. Treatment may sometimes be considered in mild subclinical hyperthyroidism with TSH levels 0.4 to 0.1 mIU/L in patients >65 years of age, especially with coexisting cardiovascular conditions, and in younger individuals only in the case of coexisting serious heart disease or symptomatic hyperthyroidism. In pregnant women, especially until the 20th week of pregnancy, subclinical hyperthyroidism should be monitored rather than treated.
Management of L-T4–induced subclinical thyrotoxicosis depends on indications for the use of this drug. In patients with thyroid cancer on TSH suppression therapy, consider L-T4 dose reduction. In subclinical thyrotoxicosis in a patient treated for hypothyroidism or a nontoxic goiter, reduce the dose of L-T4.
Pharmacotherapy can be used as the primary treatment of hyperthyroidism or as pretreatment before definitive therapy (RAI ablation or surgery).
1. Antithyroid drugs: Thionamides: The effects develop after 1 to 3 weeks of treatment (these agents inhibit synthesis of thyroid hormones but do not inhibit secretion of hormones that were synthesized earlier). Minor granulocytopenia may be a result of hyperthyroidism and is not a contraindication to the use of thionamides. If the patient has a history of agranulocytosis or liver disease, thionamides are contraindicated.
1) Methimazole (INN thiamazole): The drug of choice. The dose depends on the severity of thyrotoxicosis and varies between 2.5 and 40 mg/d orally (or bid in case of a dose ≥20 mg/d). Thyroid function tests should be monitored closely and methimazole tapered to a maintenance dose aiming to achieve euthyroidism. In severe hyperthyroidism use up to 60 mg/d orally in 2 to 3 divided doses (in the outpatient setting), and in impending thyrotoxic crisis, up to 120 mg/d orally or IV (in hospitalized patients).
In pregnant women already on methimazole, change the drug to propylthiouracil (PTU) (in the first trimester) as soon as possible (the dose ratio is ~1:20, eg, 5 mg/d thiamazole -> 100 mg/d PTU); in women treated for GD in euthyroid state disease, consider lowering the dose or discontinuation of thionamides in pregnancy. When starting treatment after 16 weeks of pregnancy or in the second trimester, methimazole is preferred at the lowest effective dose to keep the FT4 level at or just above the ULN, as the drug crosses the placenta and may affect the function of the fetal thyroid gland.
2) PTU is a second-line drug due to rare reports of serious liver injury and deaths. It should be used only in exceptional cases, such as in the first trimester of pregnancy, and in acute treatment of thyroid storm, as it blocks T4 conversion to T3. It is also used in exceptional cases in patients with allergy to methimazole in whom antithyroid treatment is required (in 50% of cases there is no cross-reactivity). The initial dose is 100 to 150 mg every 8 hours (in pregnant women 100 mg/d); the dose should be reduced after 4 to 8 weeks (euthyroidism is achieved after a longer time than with methimazole, in up to 10-17 weeks), and the maintenance dose is 50 to 150 mg/d. In pregnant women the dose should be reduced as soon as the level of FT4 approaches the ULN.
Monitoring treatment:
1) Evaluate improvement in clinical manifestations of hyperthyroidism. A rapid improvement may indicate a need for earlier reduction of doses of the antithyroid drug.
2) Measure serum TSH, FT4, and FT3 concentrations at 4 to 6 weeks of treatment; if symptoms of thyrotoxicosis have improved and serum FT4 and FT3 levels are in the lower range or below the normal limit, reduce the dose of the antithyroid drug (serum TSH levels may still be low). Normalization of serum TSH levels indicates a need for a rapid dose reduction.
3) The subsequent follow-up studies should be performed after another 4 to 6 weeks. If hyperthyroidism was not long-standing, only TSH concentrations should be measured; in patients in whom the blockade of TSH secretion was longer, this test may not be useful and management should be guided by FT4 and FT3 levels.
No regular monitoring of white blood cell (WBC) differential counts is recommended during thionamide treatment; however, it is necessary in patients with suspected granulocytopenia or agranulocytosis. If fever, sore throat, signs of infections, or mouth sores develop, the patient should discontinue thionamide and promptly seek medical attention. If granulocyte counts during treatment are 1.5×109/L to 1×109/L, more frequent follow-up is necessary and reduction of antithyroid drug dose should be considered. If granulocyte counts are 1×109/L to 0.5×109/L, the dose of antithyroid drugs should be reduced and their discontinuation considered. If granulocyte counts are <0.5×109/L, discontinuation of the antithyroid drug is mandatory (treatment with granulocyte colony-stimulating factor may be effective). Periodic monitoring of liver function enzymes during thionamide therapy may be considered, but testing is necessary with any suspicion of liver damage. The patient should be informed about the possible adverse effects of treatment, including possible liver damage (jaundice, discolored stools, dark urine), and the need to seek medical advice in the event of itching, joint pain, stomachache, nausea, or severe weakness. Women should be advised of the risk of fetal malformations in connection with antithyroid therapy. This risk can be minimized by stopping these drugs as early as possible or by using PTU in the first trimester.
Adverse effects:
1) Rare but warranting discontinuation of thionamide treatment: Agranulocytosis, aplastic anemia; acute hepatitis (propylthiouracil), obstructive jaundice (methimazole); vasculitis with antineutrophil cytoplasmic antibodies, lupus-like syndrome.
2) Not requiring immediate discontinuation of thionamides: Pruritus, rash, urticaria, sometimes very intense skin symptoms (administer antihistamines, reduce thionamide dose, or switch to another thionamide); muscle or joint pain (in case of arthritis consider discontinuation of the antithyroid drug); fever (do not use salicylates, as they block the binding of T4 to its carrier protein: thyroxine-binding globulin; advise the patient to seek prompt medical attention and obtain a complete blood count [CBC] in every case of fever with sore throat or signs of infection; if the WBC count is normal, antithyroid treatment may be continued); taste disturbances, nausea and vomiting (reduce thionamide dose and administer the drug in divided doses); minor increases in serum aminotransferase levels (use the lowest effective dose and schedule follow-up tests; discontinue the drug if alanine aminotransferase [ALT] levels increase to >3 × ULN); transient granulocytopenia or thrombocytopenia (reduce thionamide dose and schedule follow-up studies).
2. Other drugs lowering thyroid hormone levels should not be routinely administered due to risk of adverse effects. They should only be used in specialized settings for limited periods of time and in specific situations: in patients with contraindications to thionamides (eg, due to agranulocytosis); for treatment of thyrotoxic crisis (see below); in cases when rapid control of hyperthyroidism is required.
1) Iodine (inorganic) in the form of potassium iodide administered as Lugol solution or saturated solution of potassium iodide (SSKI). It is used for a few days in the treatment of thyrotoxic crisis and sometimes in the preoperative treatment of patients with GD, a coexisting vascular goiter, and no thyroid nodules. It is contraindicated in iodine-induced hyperthyroidism and in patients in whom RAI treatment is planned in the future. Also see Treatment of Thyrotoxic Crisis, below.
2) Iodinated contrast media (organic iodine; IV iohexol and oral sodium ipodate) inhibit T4 to T3 conversion and the inorganic iodine they release inhibits the synthesis and secretion of thyroid hormones; they are rarely used in treatment of thyrotoxic crisis.
3) Lithium carbonate has questionable efficacy. It decreases the secretion of thyroid hormones by inhibiting proteolysis of Tg. Also see Treatment of Thyrotoxic Crisis, below.
4) Sodium perchlorate or potassium perchlorate inhibits transport of iodine to the thyroid gland and may be used in treatment of iodine-induced hyperthyroidism. It should be used for a limited period only (<4 weeks) due to adverse effects (the most serious adverse effect is bone marrow suppression) at a dose ≤1 g/d.
5) Glucocorticoids inhibit the conversion of T4 to T3. For instance, oral dexamethasone 8 mg/d or IV hydrocortisone 300 mg/d can be used in 2 to 3 divided doses in patients in whom rapid correction of thyroid hormone levels is required (when used in combination with thionamide and inorganic iodine glucocorticoids allow for significant reduction or normalization of FT3 levels within 24-48 hours).
3. Beta-blockers: Indications include tachycardia and supraventricular arrhythmias, eyelid retraction, tremor, and hyperhidrosis. If antithyroid drugs alone are effective, beta-blockers are not required. Oral propranolol is used at doses of 10 to 40 mg tid; larger doses may be used in treatment of thyrotoxic crisis. Other oral beta1-selective drugs are usually used since they are much more convenient, especially if there is no thyroid storm: bisoprolol, metoprolol, or atenolol.
1. Effects and risks: RAI (131I) emits radiation limited to the thyroid gland. A portion of the administered 131I that has not been taken up by the thyroid gland is rapidly excreted with urine. Radiation exposure of sensitive organs (bone marrow, gonads) is low.
2. Contraindications: Pregnancy and breastfeeding; confirmed or suspected thyroid malignancy in a patient with hyperthyroidism; large thyroid goiter; patients unable to follow the recommended safety precautions, including contraception.
3. Safety precautions: Before starting treatment, make sure that female patients are not pregnant; because of radiation emitted by 131I taken up by the thyroid gland, the patient must avoid any contact with young children and pregnant women for ~7 to 10 days, depending on the dose given, to avoid causing their exposure to ionizing radiation. After completing the treatment, female patients should not become pregnant for 6 months; the recommended 6-month contraception also applies to male patients treated with 131I. There is no risk of permanent fertility impairment or congenital malformations in children; therefore, reproductive age is not a contraindication to RAI treatment. If RAI treatment is used in patients with active thyroid-associated orbitopathy, especially if moderate, concomitant prophylactic glucocorticoid therapy should be used (see below). RAI treatment should probably be avoided in severe orbitopathy.
4. Preparation for RAI treatment:
1) Discontinue methimazole or PTU 5 to 7 days before planned RAI treatment.
2) Check iodine uptake (to plan required 131I activity; thyroid radiation sensitivity differs in patients with GD and other types of hyperthyroidism).
3) Exclude pregnancy immediately before the administration of 131I (a negative pregnancy test result).
4) Advise the patient on the requirement of overnight fasting before the administration of RAI (131I is administered orally) and on subsequent management (including precautions and other radiation safety measures).
5. Management after RAI therapy: Euthyroidism is achieved within 6 weeks to 6 months after 131I administration. Some patients require continued antithyroid treatment throughout this time.
6. Indications for repeated RAI therapy: Persistent hyperthyroidism 6 months after therapy or recurrence of hyperthyroidism (10%). A final evaluation of treatment efficacy is performed at 1 year.
7. Monitoring of thyroid function is required for early detection and treatment of hypothyroidism. Measure serum TSH, FT4, and FT3 concentrations at 6 weeks; at 3, 6, and 12 months; and then TSH every 6 to 12 months for hypothyroidism. Prevention of overt hypothyroidism with early detection can avoid or decrease symptoms of GO.
Surgical Treatment (Thyroidectomy)
1. Indications:
1) Absolute: Confirmed or suspected thyroid cancer in a patient with hyperthyroidism.
2) Relative: An alternative to RAI therapy (surgery is indicated in patients with large goiters causing compression of adjacent structures; patients with allergy to thionamides or those with major adverse effects while on thionamides; and patients who have contraindication to RAI treatment, are unable to follow radiation precautions, or do not want to receive RAI therapy). Thyroidectomy by an experienced surgeon may be a life-saving procedure in thyroid storm if other therapies have been unsuccessful, resulted in significant adverse effects, or are contraindicated.
2. Preparation for surgery:
1) Elective surgery: In patients with previously untreated hyperthyroidism, administer methimazole at full doses for ≥4 to 6 weeks to achieve clinical remission of thyrotoxicosis and normalization of serum free thyroid hormone concentrations. In patients with vascular goiters, administration of Lugol solution may facilitate surgery by reducing goiter size and vascularity: give 3 to 7 drops of Lugol solution tid for 7 to 10 days before surgery. In patients with large goiters titrate the dose up to 10 to 15 drops tid; 1 to 2 drops of SSKI tid can be given instead of Lugol solution.
2) Urgent surgery may require administration of high doses of iodine and glucocorticoids, as in treatment of thyrotoxic crisis (see below).
3. Extent of surgery: Near-total thyroidectomy (leaving <1 mL of thyroid parenchyma) or total thyroidectomy are recommended for patients with GD who opted for surgical options.Evidence 4 Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of intervention). Guo Z, Yu P, Liu Z, Si Y, Jin M. Total thyroidectomy vs bilateral subtotal thyroidectomy in patients with Graves' diseases: a meta-analysis of randomized clinical trials. Clin Endocrinol (Oxf). 2013 Nov;79(5):739-46. doi: 10.1111/cen.12209. Epub 2013 Apr 19. PubMed PMID: 23521078. Less extensive procedures carry a high risk of recurrence.
4. Complications following surgery: Permanent complications (lasting >12 months) are rare (they are more frequently associated with total or repeated thyroidectomy). Temporary complications, usually lasting several weeks to months, are more common and include hypoparathyroidism (see Hypoparathyroidism) and iatrogenic injury of the recurrent laryngeal nerve with resulting vocal cord paralysis (most commonly unilateral and causing hoarseness; very rarely bilateral paralysis with serious respiratory compromise, which may require emergency tracheotomy).
5. Postoperative L-T4 replacement therapy should be started immediately after surgery (calculated dose 1.6 microg/kg/d). Start from a low dose of L-T4, for instance, 50 microg/d, or 25 microg/d in the elderly (in the initial period of treatment patients may have increased sensitivity to thyroxine), and titrate up slowly based on TSH values.
Treatment of Thyrotoxic Crisis (Thyroid Storm)
Start treatment immediately, without waiting for confirmation by laboratory tests. Continue treatment at an intensive care unit.
1. Drugs:
1) To reduce serum thyroid hormone levels:
a) Methimazole 20 to 30 mg orally qid or PTU 200 mg orally every 4 to 6 hours (PTU for acute phase only; either drug via a nasogastric tube if needed). Both drugs may be specially prepared for rectal or IV use.
b) Give iodine as soon as possible (unless thyrotoxic crisis is induced by iodine exposure) but ≥1 hour after the first dose of thionamide (to avoid utilization of iodine for synthesis of new thyroid hormones): oral SSKI 800 to 1000 mg/d in 4 divided doses (4-5 drops qid; 1 drop = 50 mg of iodine) or Lugol solution (10-30 drops bid to qid; 1 drop = 8 mg). Alternatively use IV iohexol 0.6 g (2 mL) bid or oral sodium ipodate.
c) Bile acid sequestrants (eg, cholestyramine) bind T4 in the gut, interfering with its enterohepatic circulation, and may help reduce levels of T4.
2) Beta-blockers, for instance, IV propranolol 2 mg over 2 minutes (the dose may be repeated after a few minutes), then 2 mg every 4 hours; alternatively 40 to 80 mg orally tid to qid (this also causes minor inhibition of T4 conversion to T3).
3) Hydrocortisone 50 to 100 mg IV qid (inhibits T4 conversion to T3 and treats possible associated adrenal insufficiency).
4) Antibiotics if there is a suspicion of infection (continue empiric antibiotic therapy until culture and sensitivity results are available).
5) Sedative or anticonvulsive drugs if necessary.
2. Administer oxygen as needed. Increase oxygen flow if necessary.
3. Correct water-electrolyte disturbances while monitoring volume status and assess serum electrolyte levels every 12 hours.
4. Treat hyperthermia: Use noninvasive external cooling and acetaminophen (INN paracetamol). Salicylates should be avoided as they displace T4 from thyroid hormone–binding globulin, leading to an increase in FT4.
5. Aggressively treat the precipitating condition, such as infection, ketoacidosis, pulmonary embolism, or other conditions.
6. Use thromboprophylaxis (see Primary Prevention of Venous Thromboembolism) if indicated, for instance, in atrial fibrillation, severe heart failure, or immobilization.
7. Plasmapheresis may be considered if there is no effect of treatment after 24 to 48 hours.
8. Lithium carbonate has questionable efficacy. It decreases the secretion of thyroid hormones. Oral lithium carbonate may be used in doses of 750 mg/d to 900 mg/d in the treatment of thyrotoxic crisis or sometimes in severe hyperthyroidism (particularly in patients with contraindications to thionamides). Its use is limited by adverse effects (eg, renal, neurologic).
Mortality rates in thyrotoxic crisis are 10% to 30%. Therefore, efforts should be made to prevent it with early and effective therapy of thyrotoxicosis.
ComplicationsTop
Indirect (eg, stroke in a patient with atrial fibrillation caused by hyperthyroidism) or direct and acute (thyrotoxic crisis—a life-threatening complication) or chronic (atrial fibrillation, osteoporotic fractures) consequences of the excess of thyroid hormones. The risk of persistent atrial fibrillation in hyperthyroidism is increased ~3-fold and attempts to restore normal sinus rhythm are unsuccessful until the resolution of thyrotoxicosis is achieved. Increased cardiovascular morbidity and mortality results from elevated risk of arrhythmias, thromboembolic complications associated with atrial fibrillation, and worsening of coronary heart disease or heart failure.
Tables and FiguresTop
|
Temperature |
|
|
38-38.5 degrees Celsius |
5 points |
|
38.6-39 degrees Celsius |
10 points |
|
39.1-39.5 degrees Celsius |
15 points |
|
39.6-40 degrees Celsius |
20 points |
|
40.1-40.6 degrees Celsius |
25 points |
|
>40.6 degrees Celsius |
30 points |
| Central nervous system disturbance | |
|
Absent |
0 points |
|
Mild (agitation) |
10 points |
|
Moderate (delirium, psychosis, extreme lethargy) |
20 points |
|
Severe (seizure, coma) |
30 points |
|
Gastrointestinal-hepatic dysfunction |
|
|
Absent |
0 points |
|
Moderate (diarrhea, abdominal pain, nausea/vomiting) |
10 points |
|
Severe (jaundice) |
20 points |
|
Cardiovascular dysfunction |
|
|
Tachycardia (beats/min) |
|
|
<90/min |
0 points |
|
90-109/min |
5 points |
|
110-119/min |
10 points |
|
120-129/min |
15 points |
|
130-139/min |
20 points |
|
≥140/min |
25 points |
|
Congestive heart failure |
|
|
Absent |
0 points |
|
Mild (pedal edema) |
5 points |
|
Moderate (bibasilar rales) |
10 points |
|
Severe (pulmonary edema) |
15 points |
|
Atrial fibrillation |
|
|
Absent |
0 points |
|
Present |
10 points |
|
A precipitating event in patients with untreated or inappropriately treated hyperthyroidism: acute infection, trauma, surgery, childbirth, ketoacidosis, myocardial infarction, stroke or transient ischemic attack, radioiodine treatment (rarely), or administration of iodinated contrast media |
|
|
Absent |
0 points |
|
Present |
10 points |
|
<25 points, unlikely to represent thyrotoxic crisis; 25-44 points, suggestive of impending crisis; ≥45 points, highly suggestive of thyrotoxic crisis; rapid, aggressive specific and supportive treatment required. |
|
|
Adapted from mdcalc.com. |
|
|
Criteria |
Graves disease |
Nonautoimmune hyperthyroidism (toxic MNG, solitary autonomously functioning nodule) |
|
History |
Recurrent hyperthyroidism; family history of autoimmune thyroid disease or other autoimmune diseases |
Previous nontoxic MNG |
|
Signs and symptoms of thyrotoxicosis |
No differential features | |
|
Goitera |
Features of vascular goiterb |
MNG or solitary nodule |
|
Ocular signs and symptoms |
Features of orbitopathy (immunologic inflammation), overt orbitopathy in 20%-30% of patients, severe form of progressive ophthalmopathy with infiltrates and edema in 2%-3% of patients |
Ocular signs and symptoms of sympathetic hyperactivity (lid lag and stare) do not preclude the diagnosis |
|
Pretibial myxedema |
1%-3% of patients |
None |
|
Laboratory thyroid function tests |
↓ TSH and ↑ FT4 (less commonly ↑ FT3), no differential features | |
|
TRAb |
95% of patients |
Absent |
|
↑ Anti-TPOc |
70% of patients |
15% of patients (elderly) |
|
Thyroid Doppler ultrasonography |
Diffuse vascularity of thyroid parenchymab |
Nodules |
|
Thyroid radionuclide scintigraphy |
High uptake and homogeneous concentration of tracer |
Autonomously functioning (hot) nodules and nonfunctioning (cold) areas |
|
a Lack of goiter is not a differential feature. b Nodules may be present in one-fourth of patients. c Usually not indicated as not helpful in clarifying etiology (may also be present in healthy individuals and patients with nonautoimmune thyroid diseases). | ||
|
↑, increased level; ↓, decreased level; anti-TPO, anti-thyroperoxidase; FT3, free triiodothyronine; FT4, free thyroxine; MNG, multinodular goiter; TRAb, thyroid-stimulating hormone receptor antibodies; TSH, thyroid-stimulating hormone. | ||
|
Type I |
Type II | |
|
Previous history of thyroid disease |
Multinodular goiter or Graves disease (usually undiagnosed) |
None |
|
Pathomechanism |
Excess iodine causing increased synthesis of thyroid hormones (3 mg of inorganic iodine per 100 mg of amiodarone with typical diet including <0.5 mg of iodine/d) |
Toxic effect of amiodarone (inflammation) causing damage of thyroid cells and release of thyroid hormones |
|
Iodine uptake |
>5% |
<2% |
|
Color flow thyroid Doppler ultrasonography |
Thyroid gland frequently enlarged, nodules may be present; increased blood flow |
Normal appearance of thyroid gland; significantly decreased/absent blood flow |
|
TRAb |
Increased in Graves disease |
Negative |
|
Pharmacologic treatmenta |
Eg, methimazole 40-60 mg/d + sodium perchlorate (<4 weeks) 200-250 mg qid (inhibits iodine accumulation in thyroid gland); consider radical treatment |
For instance, prednisone 40-60 mg/d for 1-3 months, then taper off the dose over another 2 months |
|
a When differential diagnosis of these types is impossible and thyroid status from before amiodarone use is unknown, combined treatment can be used: start with methimazole; add glucocorticoids if there is no improvement. | ||
|
qid, 4 times a day; TRAb, thyroid-stimulating hormone receptor antibodies. | ||
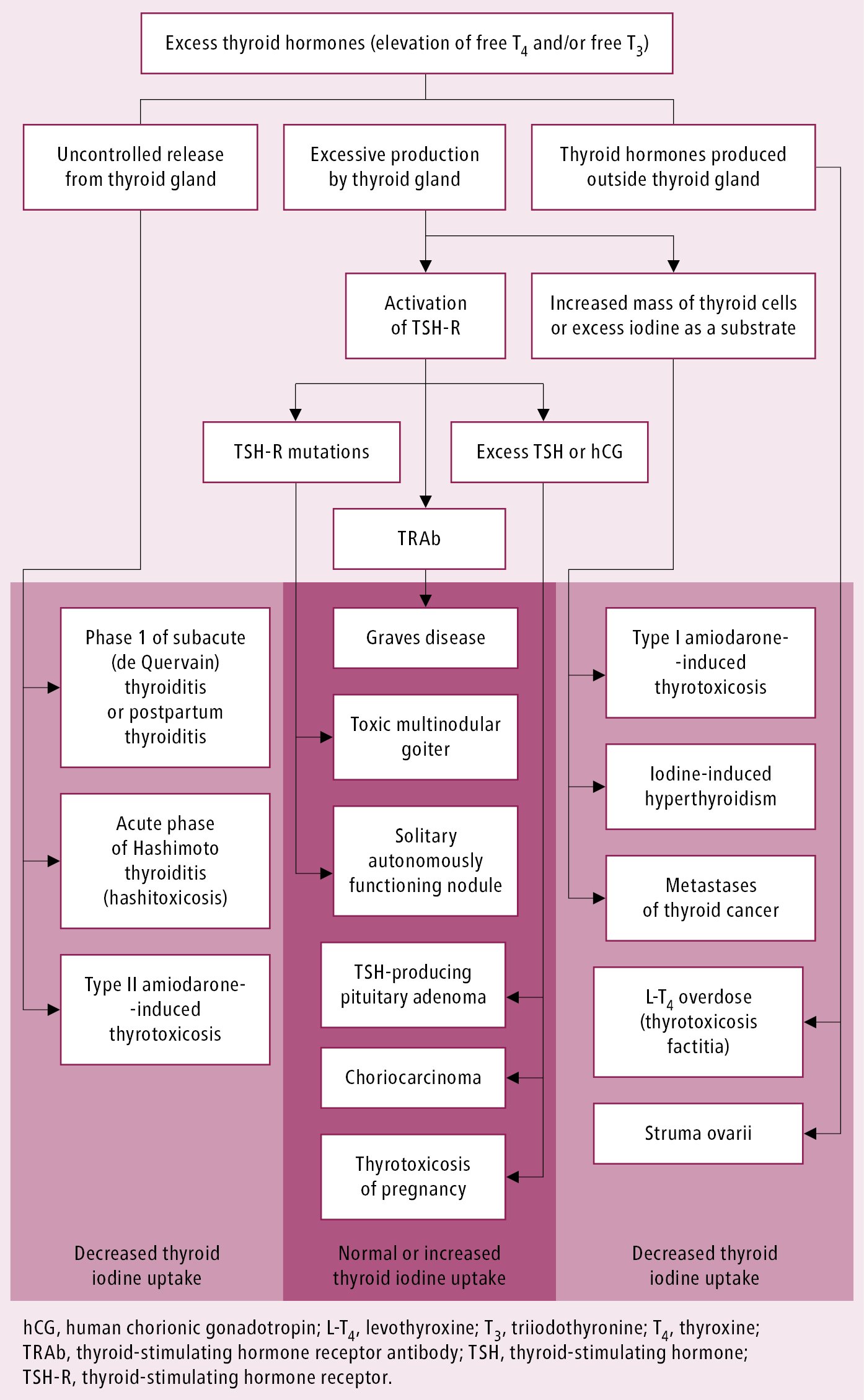
Figure 6.7-2. Causes of thyrotoxicosis.
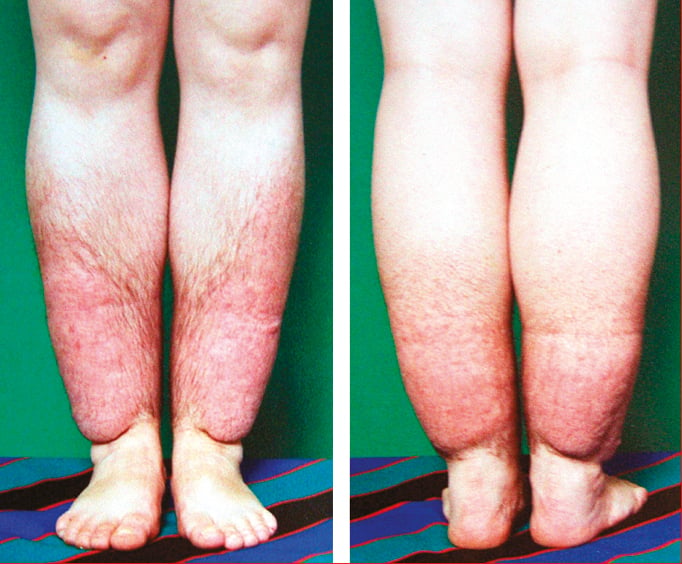
Figure 6.7-3. Thyroid dermopathy (pretibial myxedema) in Graves disease.