Qadir N, Sahetya S, Munshi L, et al. An Update on Management of Adult Patients with Acute Respiratory Distress Syndrome: An Official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2024 Jan 1;209(1):24-36. doi: 10.1164/rccm.202311-2011ST. PMID: 38032683; PMCID: PMC10870893.
Fan E, Del Sorbo L, Goligher EC, et al. An official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017 May 1;195(9):1253-1263. doi: 10.1164/rccm.201703-0548ST. PMID: 28459336. Erratum in: Am J Respir Crit Care Med. 2017 Jun 1;195(11):1540. doi: 10.1164/rccm.19511erratum. PMID: 28569586.
Invasive mechanical ventilation (IMV) is usually initiated in the operating room, emergency room, or intensive care unit, as the insertion of an endotracheal tube or laryngeal mask airway generally requires sedation and indications necessitate a monitored setting for care. Tracheostomy is more commonly used in patients requiring longer-term IMV (usually >10-14 days), but it is occasionally used as the initial form of respiratory support (eg, in those with upper airway obstruction). In selected cases of chronic irreversible respiratory failure (eg, spinal cord injury), long-term IMV, usually by tracheostomy, can also be provided at home or in a long-term care facility.
IndicationsTop
The most common indications for IMV are apnea and respiratory failure that are unlikely to be successfully managed with noninvasive approaches (simple oxygen therapy, nasal high-flow therapy [NHFT], or noninvasive ventilation [NIV]), need for airway protection in unconscious patients (eg, general anesthesia, trauma, overdose, cardiac arrest), or need to secure a definitive airway due to imminent airway loss (eg, tumor, angioedema).
Techniques and PrinciplesTop
Parameters that can be adjusted during ventilatory support: Individual parameters set independently by the clinician and those resulting from interactions from the patient’s ventilatory system vary between different modes of ventilation (see Noninvasive Mechanical Ventilation and Continuous Positive Airway Pressure). The most commonly adjusted parameters include:
1) Fraction of inspired oxygen (FiO2) delivered in the inspiratory gas mixture, expressed as a decimal fraction. FiO2 can range from 0.21 (room air) to up to 1.0 (pure oxygen), with the remainder of the inhaled gas made up of air or therapeutic gases (eg, anesthetic agents, heliox, nitric oxide).
2) Respiratory frequency (f) in respirations per minute; this may be the patient’s intrinsic rate or set using the ventilator.
3) Tidal volume (Vt) in liters; often expressed as a decimal fraction (eg, 450 mL can be reported as 0.45 L). It may be set (eg, volume control ventilation) or it may be the result of a set pressure interacting with the patient’s respiratory system (in pressure-controlled modes).
4) Positive end-expiratory pressure (PEEP) expressed in centimeters of H2O, which is the pressure at the end of the exhaled breath. A higher PEEP prevents derecruitment and facilitates maintenance of the lung surface area for gas exchange.
5) Inspiratory pressure, which is the pressure during inspiration. Inspiratory pressure resulting from IMV depends on the flow and volume of the delivered gas and characteristics of the patient’s respiratory system. Peak inspiratory pressures (PIPs) occur at maximal inhalation and reflect both the resistance and elastance of the respiratory system. Plateau pressure (Pplat), obtained during an inspiratory hold, reflects only the elastance of the system (since the flow is 0, there is no resistance component). Depending on the mode of ventilation, the inspiratory pressure may be set by the clinician (independent) or depend on a set volume (Table 21.15-1; Figure 21.15-1). Inspiration may be spontaneous (triggered by the patient) or set (triggered by the ventilator).
6) Inspiratory time (Ti), expressed in seconds, determines the ratio of inspiratory to expiratory time (I:E). It is sometimes set directly on the ventilator or may be determined by the flow, which drops as inspiration proceeds during a fixed-pressure breath.
7) Trigger sensitivity, which is the flow or pressure that must be generated by the patient to trigger a supported breath. It varies between modes.
8) Humidification, which can be provided using either active heated humidifiers or a heat and moisture exchanger (HME). It may increase patient comfort and can assist in secretion management.
Mechanical Ventilation ModesTop
There are many different modes that can be used in IMV (names may vary between device manufacturers):
1) Continuous mandatory ventilation (CMV): In this mode all breaths are initiated and delivered entirely by the ventilator, in accordance with the parameters set by the clinical team. In general, either pressure control (PC) or volume control (VC) can be used. In PC the pressure is set and the Vt varies with changes in the patient’s respiratory compliance. In VC the Vt is set and the pressures vary with changes in the patient’s respiratory compliance.
2) Assist-control (AC) ventilation: This mode is identical to CMV, but if the patient attempts to trigger a breath sooner than the set respiratory rate, the ventilator delivers an additional fully supported breath in the same manner (PC or VC).
3) Pressure support ventilation (PSV): The patient breathes spontaneously and the ventilator provides supported breaths (initiated by the patient) at a set pressure. The breath is completed when a set flow parameter (eg, 25% of peak inspiratory flow) is reached, indicating that the patient’s inspiratory effort is ending. For safety, the ventilator has a backup rate and provides fully supported breaths if the patient fails to trigger a breath after a set time.
4) Synchronized intermittent mandatory ventilation (SIMV): The ventilator delivers fully supported breaths at a set rate, similarly to CMV, but if the patient triggers a breath in between, a supported breath, similar to PSV, is delivered. This mode is used infrequently.
5) Proportional pressure support (PPSV) or proportional assist ventilation (PAV): The pressure support delivered is proportional to the work of breathing (greater effort of the patient increases inspiratory pressure).
6) Adaptive support ventilation (ASV) adjusts the frequency of breathing and inspiratory pressure to the breathing mechanics of the patient.
7) Neurally adjusted ventilatory assist (NAVA): The respiratory rate and amount of support are based on the electromyographic signal from the diaphragm.
8) Airway pressure release ventilation (APRV): This includes a prolonged period at high airway pressure with short intermittent periods at a lower pressure. The pressure drop facilitates exhalation and carbon dioxide elimination. APRV is rarely used.
9) Pressure-limited ventilation with guaranteed volume (pressure-regulated volume control [PRVC]) or pressure control volume guarantee (average volume–assured pressure support [AVAPS]): These modes adapt to changes in the respiratory system, either mechanical or in the patient’s effort. Based on the analysis of previous respiratory cycles, the ventilator selects the lowest inspiratory pressure to achieve the set Vt or minute ventilation (MV).
Special Ventilation Techniques Top
1. Prone ventilation can improve blood oxygenation through aeration of the dorsal, usually hypoventilated or atelectatic, lung areas. It is used in patients with acute respiratory distress syndrome (ARDS) for 12 to 16 hours per day when the partial pressure of arterial oxygen (PaO2)/FiO2 ratio is ≤150 mm Hg. It may require up to 4 to 6 clinicians who are skilled in the technique to safely pronate and supinate a patient.Evidence 1Strong recommendation (benefits likely outweigh downsides; right action for all or almost all patients). Moderate Quality of Evidence (moderate confidence that we know the true effects of the intervention). Quality of Evidence lowered due to imprecision of estimate. Fan E, Del Sorbo L, Goligher EC, et al. An official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017 May 1;195(9):1253-1263. doi: 10.1164/rccm.201703-0548ST. PMID: 28459336. Erratum in: Am J Respir Crit Care Med. 2017 Jun 1;195(11):1540. doi: 10.1164/rccm.19511erratum. PMID: 28569586.
2. Alveolar recruitment maneuvers use a temporary short-term increase in the airway positive pressure to high values in order to recruit collapsed alveoli. The maneuvers are used when atelectasis plays a role in hypoxic respiratory failure; while use in some patients with ARDS may be helpful, prolonged recruitment maneuvers (PEEP ≥35 cm H2O for >60 s) should be avoided.Evidence 2Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). Low Quality of Evidence (low confidence that we know the true effects of the intervention). Quality of Evidence lowered due to imprecision of estimate and indirectness, as most trials included recruitment maneuvers as part of a varied “open lung” bundle. Fan E, Del Sorbo L, Goligher EC, et al. An official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017 May 1;195(9):1253-1263. doi: 10.1164/rccm.201703-0548ST. PMID: 28459336. Erratum in: Am J Respir Crit Care Med. 2017 Jun 1;195(11):1540. doi: 10.1164/rccm.19511erratum. PMID: 28569586.
3. Inhalation of nitric oxide (NO) or prostacyclin in addition to conventional ventilation in refractory hypoxemia dilates pulmonary vessels in better ventilated areas, thus improving blood flow within them and increasing their oxygenation. However, as this does not translate into a mortality benefit and may cause renal dysfunction, it is not recommended for routine use but may be considered as rescue therapy.Evidence 3Weak recommendation (downsides likely outweigh benefits, but the balance is close or uncertain; an alternative course of action may be better for some patients). Moderate Quality of Evidence (moderate confidence that we know the true effects of the intervention). Quality of Evidence lowered due to imprecision. Adhikari NKJ, Burns KEA, Friedrich JO, Granton JT, Cook DJ, Meade MO. Effect of Nitric Oxide on Oxygenation and Mortality in Acute Lung Injury: Systematic Review and Meta-Analysis. BMJ. 2007 Apr 14;334(7597):779. doi: 10.1136/bmj.39139.716794.55. Epub 2007 Mar 23. PMID: 17383982; PMCID: PMC1852043.
4. Independent lung ventilation (ILV) requires the use of a bilumen endotracheal tube (ETT) and 2 ventilators. ILV can be considered when lesions dominate in one lung and may allow lower pressures to be applied to the less affected or healthy lung, thereby preventing ventilator-associated lung injury (VILI). Data for this rarely used technique are scarce.
5. Extracorporeal membrane oxygenation (ECMO) requires large-bore arterial and venous cannulation and allows for gas exchange to occur outside the lungs. Venovenous ECMO is used for severe to refractory ARDS and requires specialized centers with adequate training, expertise, and equipment (see Extracorporeal Membrane Oxygenation).
Principles of UseTop
Below we outline the general principles of IMV, although respiratory needs vary in specific circumstances (eg, patients with airflow obstruction, restrictive lung disease, or bronchopleural fistulae).
1. Correct hypoxemia while keeping oxygen content in the inspiratory mixture as low as possible to maintain target oxygen saturation (generally ≤96%; oxygen therapy targets: see Oxygen Therapy).
2. Use PEEP to maintain alveolar recruitment at the end of exhalation and optimize the surface area for gas exchange, generally set according to an optimal PEEP study (eg, decremental PEEP strategy) or the PEEP/FiO2 table. In patients with moderate to severe ARDS, a higher PEEP strategy may be preferred.Evidence 4Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). Moderate Quality of Evidence (moderate certainty that we know the true effect of the intervention). Quality of Evidence lowered due to imprecision. Walkey AJ, Del Sorbo L, Hodgson CL, et al. Higher PEEP Versus Lower PEEP Strategies for Patients with Acute Respiratory Distress Syndrome. A Systematic Review and Meta-Analysis. Ann Am Thorac Soc. 2017 Oct;14(Supplement_4):S297-S303. doi: 10.1513/AnnalsATS.201704-338OT.
3. Carefully correct hypercapnia by increasing the MV (f × Vt) while maintaining lung-protective ventilation.
4. Lung-protective ventilation aims to avoid iatrogenic VILI. Target Vt of 4 to 6 mL per kg of ideal body weight and set respiratory rate (RR) to maintain sufficient MV (RR of up to 35/min may be needed). This may be difficult to achieve and some recent ARDS trials report a mean Vt slightly >6 mL/kg, even in controlled research setting (if drive pressure can be minimized). Lung-protective ventilation may also necessitate tolerating permissive hypercapnia with pH >7.2. Other targets include Pplat (during inspiratory pause) <30 cm H2O. COVID-19 management: see Coronavirus Disease 2019 (COVID-19).
5. Controlled, systematic, and stepwise withdrawal of mechanical ventilation including the use of spontaneous breathing trials (SBTs): Once patients have an adequate neurologic status, there is improvement of acute metabolic disorders, hemodynamic stability, manageable secretion, and improvement in oxygenation and ventilatory function (usually FiO2 ≤0.40 and PEEP typically ≤5-8 cm H2O), they should be assessed for extubation at least daily using an SBT. Most commonly this is done with either a t-piece or low PSV (eg, 5/5) for 30 minutes. Patients “fail” the test and resume IMV in the event of fatigue or agitation, disturbance of consciousness, acceleration and shallowness of breathing, intensification of gasometric abnormalities, or circulatory instability. Patients who successfully complete a 30-minute SBT are generally extubated unless there are specific reasons not to.
6. After withdrawal of IMV, noninvasive ventilationEvidence 5Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). Moderate Quality of Evidence (moderate certainty that we know the true effect of the intervention). Quality of Evidence lowered due to imprecision. Oczkowski S, Ergan B, Bos L, et al. ERS Clinical Practice Guidelines: High-flow nasal cannula in acute respiratory failure. Eur Respir J. 2021 Oct 28;2101574. doi: 10.1183/13993003.01574-2021. PMID: 34649974.Rochwerg B, Einav S, Chaudhuri D, et al. The role for high flow nasal cannula as a respiratory support strategy in adults: a clinical practice guideline. Intensive Care Med. 2020 Dec;46(12):2226-2237. doi: 10.1007/s00134-020-06312-y. Epub 2020 Nov 17. PMID: 33201321; PMCID: PMC7670292. (see Noninvasive Mechanical Ventilation and Continuous Positive Airway Pressure) and/or high-flow nasal oxygen therapy (HFNOT)Evidence 6Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). Moderate Quality of Evidence (moderate certainty that we know the true effect of the intervention). Quality of Evidence lowered due to imprecision. Oczkowski S, Ergan B, Bos L, et al. ERS Clinical Practice Guidelines: High-flow nasal cannula in acute respiratory failure. Eur Respir J. 2021 Oct 28;2101574. doi: 10.1183/13993003.01574-2021. PMID: 34649974. Rochwerg B, Einav S, Chaudhuri D, et al. The role for high flow nasal cannula as a respiratory support strategy in adults: a clinical practice guideline. Intensive Care Med. 2020 Dec;46(12):2226-2237. doi: 10.1007/s00134-020-06312-y. Epub 2020 Nov 17. PMID: 33201321; PMCID: PMC7670292. (see Oxygen Therapy) can be used to increase the success of extubation.
ComplicationsTop
1. VILI, caused by numerous mechanisms including:
1) Barotrauma of the alveoli: Manifested as pneumothorax, pneumomediastinum, or worsening respiratory status; induced by ventilation parameters (eg, excessive PEEP); patient-ventilator dyssynchrony (resulting in transient but excessive swings in airway and alveolar pressure); obstruction (eg, gas trapping/hyperinflation); stiff or fibrotic lungs.
2) Volutrauma due to excessive Vt and distention of the alveoli.
3) Atelectrauma, caused by alternation of collapse and opening (aeration) of the airways and alveoli, associated with too low PEEP, Vt, or both.
4) Biotrauma (damage by biologic mediators released from stressed lungs during mechanical ventilation).
2. Hypotension and organ hypoperfusion: Positive intrathoracic pressure from IMV causes decreased venous return and reduced cardiac preload.
3. Venous stasis and increase in intracranial pressure due to positive intrathoracic pressure impairing venous return.
4. Ventilator-associated pneumonia (VAP) related to the presence of the ETT. VAP prevention “bundles” are commonly used, which incorporate elevation of the bed head to 30 to 45 degrees to reduce aspiration; oral or selective gut decontamination (or both); and use of ETTs with subglottic suction ports, which remove oral secretions before they pass into the lungs.
5. Posthypercapnic alkalosis caused by excessive MV in patients with partially or fully compensated respiratory acidosis.
6. Complications due to ETT use, including tracheal ulcer, tracheal stenosis, and vocal cord dysfunction. These can be reduced by maintaining only the minimum sealing pressure in the endotracheal cuffing that is needed to maintain tube tightness (usually >20 cm H2O to avoid leakage and aspiration) and using the smallest sufficiently sized tube.
7. Neuromuscular weakness, a common complication after IMV that can result in reduced mobility and inability to complete activities of daily living (ADLs) and instrumental activities of daily living (IADLs). Dysphagia is also common in patients requiring ventilation for more than a few days.
Tables and FiguresTop
|
Characteristics |
Limited-volume ventilation |
Limited-pressure ventilation |
|
Key adjustable parameter |
Vt |
Peak inspiratory pressure |
|
Key monitored parameter |
Peak inspiratory pressure |
Vt, MV |
|
Main uses |
CNS disorders, general anesthesia |
Lung diseasesa |
|
a Pressure-limited ventilation is frequently preferred due to pathophysiologic considerations (preventing a barotrauma requires less vigilance than with volume-limited ventilation). |
||
|
CNS, central nervous system; MV, minute ventilation; Vt, tidal volume. |
||
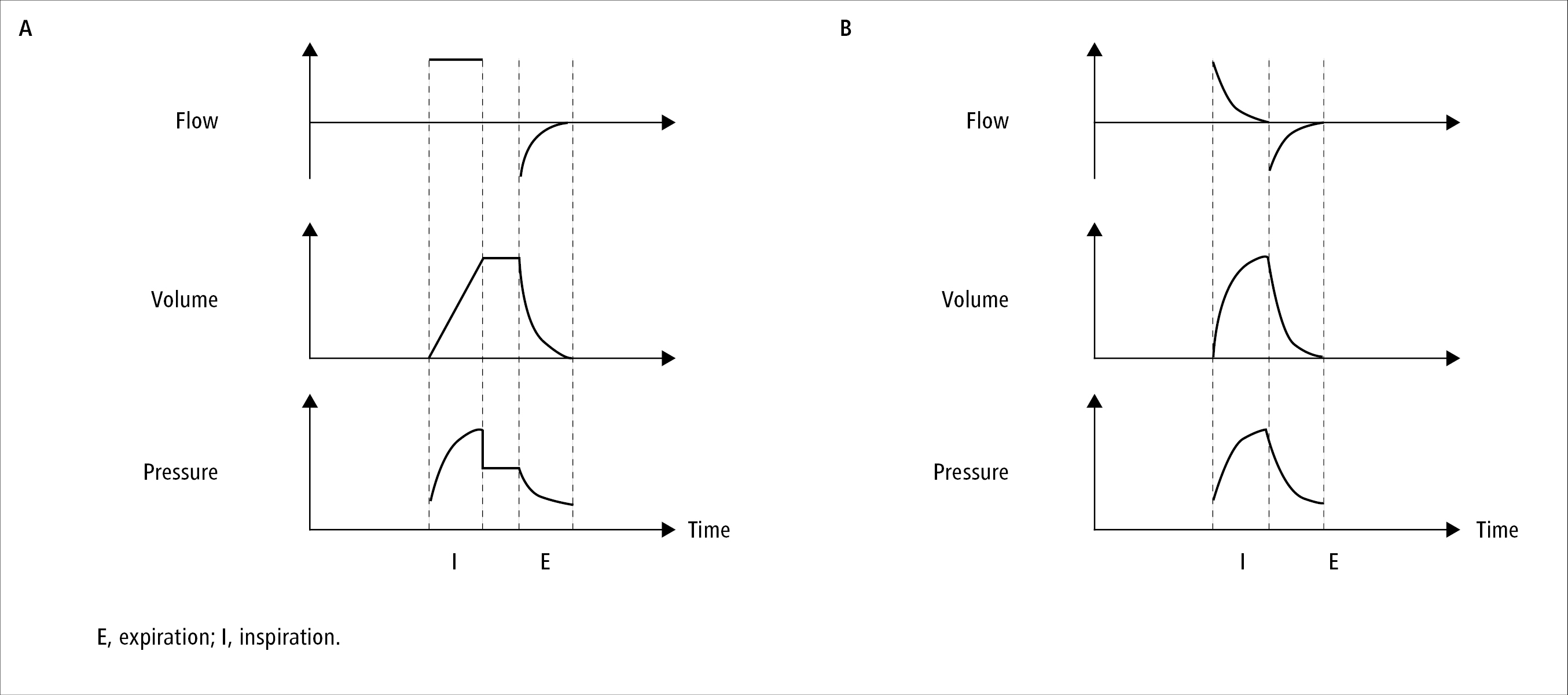
Figure 21.15-1. Relationship between time and gas flow, lung volume, and airway pressure during volume-limited ventilation with an inspiratory pause (A) and pressure-limited ventilation without an inspiratory pause (B). Positive end-expiratory pressure (PEEP) used (airway pressure >0). Inspiratory pause pressure referred to as plateau pressure.