Shoamanesh A, Patrice Lindsay M, Castellucci LA, et al. Canadian stroke best practice recommendations: Management of Spontaneous Intracerebral Hemorrhage, 7th Edition Update 2020. Int J Stroke. 2021 Apr;16(3):321-341. doi: 10.1177/1747493020968424. Epub 2020 Nov 11. PMID: 33174815.
National Institute of Neurological Disorders and Stroke. National Institutes of Health Stroke Scale. https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Preventing-Stroke/Stroke-Scales-and-Related-Information. Accessed September 12, 2019.
Boulanger JM, Lindsay MP, Gubitz G, et al. Canadian Stroke Best Practice Recommendations for Acute Stroke Management: Prehospital, Emergency Department, and Acute Inpatient Stroke Care, 6th Edition Update 2018. Int J Stroke. 2018 Dec;13(9):949-984. Epub 2018 Jul 18. PMID: 30021503.
Brigo F, Lattanzi S, Zelano J, et al. Randomized controlled trials of antiepileptic drugs for the treatment of post-stroke seizures: A systematic review with network meta-analysis. Seizure. 2018 Oct;61:57-62. doi: 10.1016/j.seizure.2018.08.001. Epub 2018 Aug 3. Review. PMID: 30096625.
Powers WJ, Rabinstein AA, Ackerson T, et al; American Heart Association Stroke Council. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2018 Mar;49(3):e46-e110. doi: 10.1161/STR.0000000000000158. Epub 2018 Jan 24. Review. Erratum in: Stroke. 2018 Mar;49(3):e138. Stroke. 2018 Apr 18. PMID: 29367334.
Catanese L, Perera KS, Ntaios G, Connolly SJ. Embolic Stroke of Undetermined Source: A Systematic Review and Clinical Update. Stroke. 2017 Apr;48(4):867-872. doi: 10.1161/STROKEAHA.116.016414. Epub 2017 Mar 6. Review. PMID: 28265016.
Hemphill JC 3rd, Greenberg SM, Anderson CS, et al; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2015 Jul;46(7):2032-60. doi: 10.1161/STR.0000000000000069. Epub 2015 May 28. PMID: 26022637.
Kernan WN, Ovbiagele B, Black HR, et al; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014 Jul;45(7):2160-236. doi: 10.1161/STR.0000000000000024. Epub 2014 May 1. Erratum in: Stroke. 2015 Feb;46(2):e54. PMID: 24788967.
Lansberg MG, O'Donnell MJ, Khatri P, et al; American College of Chest Physicians. Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012 Feb;141(2 Suppl):e601S-36S. doi: 10.1378/chest.11-2302. PMID: 22315273; Central PMCID: PMC3278065.
Whitlock RP, Sun JC, Fremes SE, Rubens FD, Teoh KH; American College of Chest Physicians. Antithrombotic and thrombolytic therapy for valvular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012 Feb;141(2 Suppl):e576S-600S. doi: 10.1378/chest.11-2305. PMID: 22315272; PMCID: PMC3278057.
Ha L, Hauge T, Spenning AB, Iversen PO. Individual, nutritional support prevents undernutrition, increases muscle strength and improves QoL among elderly at nutritional risk hospitalized for acute stroke: a randomized, controlled trial. Clin Nutr. 2010 Oct;29(5):567-73. doi: 10.1016/j.clnu.2010.01.011. Epub 2010 Feb 21. PMID: 20176418.
Duncan PW, Zorowitz R, Bates B, et al. Management of Adult Stroke Rehabilitation Care: a clinical practice guideline. Stroke. 2005 Sep;36(9):e100-43. PMID: 16120836.
Prevention
Gladstone DJ, Lindsay MP, Douketis J, et al. Canadian Stroke Best Practice Recommendations: Secondary Prevention of Stroke Update 2020. Can J Neurol Sci. 2021 Jun 18;1-23. doi: 10.1017/cjn.2021.127. Online ahead of print. PMID: 34140063.
Definition, Etiology, Pathogenesis Top
Stroke is defined as an abrupt onset of focal brain, spinal cord, or retinal injury due to abnormalities of cerebral blood flow with clinical symptoms or signs lasting >24 hours and/or radiologic abnormalities of infarction or hemorrhage present on imaging studies. Subarachnoid hemorrhage (SAH) is also considered in the category of stroke although cerebral dysfunction is usually generalized.
Focal neurologic signs and symptoms that resolve spontaneously within 24 hours and without radiologic abnormalities are referred to as a transient ischemic attack (TIA).
On the basis of pathomechanism and etiology, stroke can be classified as:
1) Ischemic stroke (accounting for ~80% of stroke cases), which is usually due to an occlusion (often a transient occlusion with embolic strokes) of an artery and the resulting reduction of focal cerebral perfusion. Ischemic stroke may be caused by:
a) Atherosclerotic plaques (in situ plaque rupture with occlusion or artery-to-artery embolism) in the major arteries supplying the brain (aortic arch, carotid and vertebral arteries) or in large- and medium-size intracranial arteries (large artery atherosclerosis).
b) Degenerative lesions/microatherothrombosis (in situ occlusion) in small penetrating arteries of the brain (lacunar stroke [LACI]).
c) Cardiac embolism from atrial fibrillation (most frequently), ischemic heart disease (left ventricular aneurysm or cardiomyopathy), mitral or aortic valvular heart disease (rheumatic valvular disease, endocarditis, or artificial bioprosthetic or mechanical valves).
d) Less common causes, such as patent foramen ovale (PFO) (paradoxical embolism), arterial dissection, or coagulopathies.
Despite recent diagnostic advances, ~17% of ischemic strokes are found to be cryptogenic (embolic stroke of undetermined source) after diagnostic evaluation.
2) Hemorrhagic stroke (accounting for ~15%-20% of stroke cases), which may be caused by:
a) Intracerebral hemorrhage (ICH): Most commonly occurs due to bleeding from a ruptured penetrating intracranial vessel in the context of hypertension (weakened vessel due to degenerative changes and microaneurysmal formation), bleeding from rupture of a lobar vessel in the elderly (weakened vessel due to amyloid deposition), or rupture from an arteriovenous malformation.
b) SAH (~5% of stroke cases): Most frequently occurs as a result of rupture of a saccular aneurysm at the base of the brain in the subarachnoid space and presents clinically with generalized brain dysfunction (headache, decreased level of consciousness [LOC]).
Hemorrhagic transformation of ischemic infarction occurs in 7% of patients after thrombolysis, but it can occur spontaneously in patients with cardioembolic stroke and large infarction. Hemorrhagic infarction is often asymptomatic because it is due to bleeding into the already damaged tissue, but if severe, it can manifest clinically as deterioration in neurologic function and is managed as spontaneous ICH.
3) Cerebral venous thrombosis (<1% of stroke cases) occurs as a result of thrombosis of the intracranial venous sinuses, deep venous system, and cortical veins, causing cerebral ischemia, infarction, and hemorrhage. The clinical presentation can be variable and includes headache, seizures, focal signs, and decreased LOC.
Risk factors: Hypertension, diabetes mellitus, dyslipidemia, tobacco smoking, physical inactivity, cardiac diseases, alcohol abuse, oral contraceptives, hormonal replacement therapy, pregnancy, migraine.
Clinical Features and Natural History Top
1. Symptoms of ischemic stroke depend on the location of the lesion. The Oxford classification identifies ischemic stroke subtypes based on location and vascular territory:
1) Total anterior circulation infarct (TACI) involves the area supplied by the anterior and middle cerebral arteries and causes total anterior circulation syndrome (TACS): significant hemiparesis or sensory disturbances affecting one side of the body in ≥2 out of 3 areas (face, upper extremities, lower extremities), aphasia, and homonymous hemianopsia.
2) Partial anterior circulation infarct (PACI) involves part of the anterior cerebral circulation and causes partial anterior circulation syndrome (PACS): motor or sensory symptoms in 1 or 2 out of the 3 areas mentioned above, or isolated aphasia.
3) LACI develops in the areas supplied by the penetrating arteries, most frequently in the basal ganglia, internal capsule, thalamus, or brainstem, and causes lacunar stroke syndrome (LACS), which is usually limited to paresis or sensory disturbances in 2 out of 3 areas (face, upper extremities, lower extremities). Isolated weakness involving all of the face, arm, and leg (pure motor stroke) is a relatively specific LACS.
4) Posterior circulation infarct (POCI) affects the vertebrobasilar system and manifests with cerebellar, brainstem, or occipital lobe signs and symptoms (posterior circulation syndrome [POCS]), including ataxia and nystagmus, cranial nerve palsies with contralateral motor or sensory deficits (or both), or isolated homonymous hemianopia.
Symptoms of hemorrhagic stroke depend on the type and location of bleeding:
1) ICH results in a variety of focal deficits depending on the location of bleeding. It is typically associated with a prominent headache due to intracranial hypertension, but the headache does not usually have a thunderclap quality. It is not possible to differentiate it on clinical grounds from cerebral infarction and imaging is required.
2) SAH should always be carefully excluded in each patient with a sudden and intense (thunderclap) headache (often described as “the worst ever in my life”). Usually, there are few focal neurologic signs, but more commonly, a change in the LOC is seen.
Cerebral venous thrombosis causes a wide range of clinical manifestations, and the area of injury does not correspond to the area of arterial blood supply. Cerebral venous thrombosis may lead to focal neurologic symptoms or partial seizures, symptoms of increased intracranial pressure, and altered mental status; and with involvement of the cavernous sinus, to abnormal eye movement (palsies of cranial nerves III and VI) with accompanying exophthalmos, retrobulbar pain, and eyelid edema.
2. Natural history: In the first hours or days following a stroke, the patient’s neurologic status may change. Up to 20% of patients with cerebral infarction will have worsening of their impairments in the first 24 to 48 hours (stroke in progression). In 5% to 10% of patients with ischemic stroke, a second stroke occurs early (new neurologic symptoms develop; it may be associated either with a new or the same blood supply area as the first stroke). In up to 10% of large cerebral infarctions (TACIs), hemorrhagic transformation may occur, usually in the first 48 hours, with possible neurologic worsening. In addition, large cerebral infarctions (TACIs) may show clinical worsening or decreased LOC due to cerebral edema between days 3 and 5. In the absence of these complications, most patients start to improve after the initial few days and the majority of motor recovery occurs over the first 2 to 3 months. The risk of stroke is ~10% in the first 30 days after a TIA, with half of the events occurring in the first few days. Rapid identification of stroke etiology and implementation of risk-reduction strategies in patients with an acute TIA are imperative.Evidence 1Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of the intervention). Rothwell PM, Giles MF, Chandratheva A, et al; Early use of Existing Preventive Strategies for Stroke (EXPRESS) study. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007 Oct 20;370(9596):1432-42. Erratum in: Lancet. 2008 Feb 2;371(9610):386. Carasco-Alexander, Faye [corrected to Alexander, Faye C]. PMID: 17928046. Lavallée PC, Meseguer E, Abboud H, et al. A transient ischaemic attack clinic with round-the-clock access (SOS-TIA): feasibility and effects. Lancet Neurol. 2007 Nov;6(11):953-60. PMID: 17928270. Hao Q, Tampi M, O'Donnell M, Foroutan F, Siemieniuk RA, Guyatt G. Clopidogrel plus aspirin versus aspirin alone for acute minor ischaemic stroke or high risk transient ischaemic attack: systematic review and meta-analysis. BMJ. 2018 Dec 18;363:k5108. doi: 10.1136/bmj.k5108. PMID: 30563866; PMCID: PMC6298178.
DiagnosisTop
1. Take a history: Carefully establish the exact time of the onset of symptoms, as this is crucial for determining indications for thrombolytic treatment or endovascular clot retrieval. Almost all stroke patients present with sudden and focal neurologic signs and symptoms.
2. Assess the vital signs (airway, breathing, circulation [ABC]): Respiration rate, blood pressure, heart rate (including electrocardiography [ECG]), and oxygen saturation (SaO2) (pulse oximetry).
3. Perform an efficient general examination: Look for evidence of trauma or other signs that may require immediate medical intervention. In patients with SAH symptoms and signs of meningeal irritation (stiff neck) can be present.
4. Perform a focused neurologic examination: A standardized stroke scale, such as the National Institutes of Health Stroke Scale (NIHSS) (Table 1), is a tool that can quantify the degree of impairment, identify stroke location, quantify change in neurologic impairments, and predict the outcome. The NIHSS is useful in determining prognosis after stroke and has become a common and reliable communication tool among clinicians.Evidence 2High Quality of Evidence (high confidence that we know true effects of the intervention). Adams HP Jr, Davis PH, Leira EC, et al. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: A report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology. 1999 Jul 13;53(1):126-31. PMID: 10408548. Lyden P, Raman R, Liu L, Emr M, Warren M, Marler J. National Institutes of Health Stroke Scale certification is reliable across multiple venues. Stroke. 2009 Jul;40(7):2507-11. doi: 10.1161/STROKEAHA.108.532069. Epub 2009 Jun 11. PMID: 19520998; PMCID: PMC2726278.
5. Assess blood glucose levels. Patients with hypoglycemia can present with a focal stroke-like picture.
6. Collect blood samples for a complete blood count (CBC), international normalized ratio (INR), activated partial thromboplastin time (aPTT), serum electrolytes, renal function tests, and serum biomarkers of myocardial infarction. Measure thrombin time (TT) if the patient is suspected to be treated with a direct thrombin inhibitor or ecarin clotting time (ECT) if a direct factor Xa inhibitor is suspected. In selected patients there may be specific indications to order liver function tests, toxicology screen, blood alcohol level, and pregnancy test.
7. Perform nonenhanced computed tomography (NECT) or magnetic resonance imaging (MRI) of the brain as soon as possible; these studies accurately differentiate ischemic stroke (Figure 1) from hemorrhagic stroke (Figure 2), which cannot be reliably done on clinical grounds, and are crucial for both acute and chronic management. While MRI offers better resolution, NECT takes a shorter time to complete, can be performed in patients with metal implants, is less susceptible to motion artifacts, and in most cases provides enough information to make emergency treatment decisions. To decide about potential clot retrieval, computed tomography angiography (CTA) or magnetic resonance angiography (MRA) is subsequently necessary to identify the presence of a large vessel in patients who are potential candidates for endovascular clot retrieval for large vessel occlusion (carotid artery, middle cerebral artery, anterior cerebral artery, or basilar artery).
The sensitivity of computed tomography (CT) scanning in the first few hours after SAH is almost 100%. If the results of head CT scanning are normal or inconclusive and SAH is suspected based on clinical presentation, perform a diagnostic lumbar puncture. Do not perform lumbar puncture until you have excluded an intracranial space-occupying lesion with brain CT or MRI.
8. Further studies should be guided by the need to identify the pathomechanism of stroke: carotid ultrasonography and/or CTA/MRA for large vessel atherosclerosis and stenosis; echocardiography (transthoracic and/or transesophageal) for a cardioembolic source; prolonged cardiac monitoring for suspected atrial fibrillation. Other studies are guided by the clinical indication: chest radiography for suspected aspiration pneumonia or electroencephalography (EEG) for suspected epileptic disorders mimicking stroke.
Differential diagnosis includes a broad spectrum of diseases that may occasionally present with and produce focal neurologic symptoms or signs. Even in expert hands >10% of patients diagnosed with ischemic stroke at the initial evaluation are found to have another disorder (sometimes labeled a stroke mimic), commonly vestibular dysfunction (23%), toxic/metabolic abnormalities (13%), seizures (13%), migraine (8%), or functional and somatization disorders including psychosomatic conversion (10%).Evidence 3Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due to indirectness. Merino JG, Luby M, Benson RT, et al. Predictors of acute stroke mimics in 8187 patients referred to a stroke service. J Stroke Cerebrovasc Dis. 2013 Nov;22(8):e397-403. doi: 10.1016/j.jstrokecerebrovasdis.2013.04.018. Epub 2013 May 13. PMID: 23680681; PMCID: PMC3812364.
Actual stroke is sometimes misdiagnosed as a different condition, usually when the temporal profile of onset is more gradual and focal deficits are initially absent or not obvious. Common misdiagnoses include reduced level of consciousness (31%), syncope (16%), hypertensive emergency (13%), or systemic infection (11%).Evidence 4Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due to indirectness. Dupre CM, Libman R, Dupre SI, Katz JM, Rybinnik I, Kwiatkowski T. Stroke chameleons. J Stroke Cerebrovasc Dis. 2014 Feb;23(2):374-8. doi: 10.1016/j.jstrokecerebrovasdis.2013.07.015. Epub 2013 Aug 15. PMID: 23954604. The existing triage scores, including FAST, BE-FAST, and ROSIER (mdcalc.com, raaems.org), have a satisfactory combination of sensitivity and specificity but serve as early warning and educational tools.
CT or MRI brain scanning reliably differentiates ischemia or infarction from hemorrhage. Most mass lesions, such as tumor, subdural hematoma, or abscess, are evident on brain imaging. Toxic and metabolic disorders simulating stroke become evident from their atypical presentation, examination, laboratory analyses, and progression of the disorder.
Treatment Top
Stroke is a medical emergency, a life-threatening condition that often results in severe and permanent neurologic impairment and disability. Therefore, it requires urgent diagnosis and immediate treatment. A patient with suspected stroke should be urgently transported to a hospital that is experienced in providing stroke care, and preferably directly to a hospital with a dedicated stroke unit.Evidence 5Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of the intervention). Langhorne P, Ramachandra S; Stroke Unit Trialists’ Collaboration. Organised inpatient (stroke unit) care for stroke: network meta‐analysis. Cochrane Database of Systematic Reviews. Cochrane Database Syst Rev. 2020 Apr 23;4(4):CD000197. doi: 10.1002/14651858.CD000197.pub4. PMID: 32324916; PMCID: PMC7197653.
1. Control the vital parameters: Immediately assess and stabilize airway, breathing, and circulation (ABC). Patients with stroke may require mechanical ventilation and treatment of cardiac dysfunction in an intensive care unit (ICU) setting.
2. Control blood pressure: In the early phase of stroke blood pressure is frequently elevated and then spontaneously decreases after a few days. An excessive lowering of blood pressure may lead to a reduction in cerebral blood flow, resulting in expansion of the ischemic lesion and deterioration of the patient’s neurologic status. Avoid rapid lowering of blood pressure.
1) Usual indications for the use of antihypertensive drugs:
a) Patients with ischemic stroke eligible for thrombolysis: Systolic blood pressure (SBP) >185 mm Hg or diastolic blood pressure (DBP) >110 mm Hg (Table 2).
b) Patients with ischemic stroke not eligible for thrombolysis: SBP >220 mm Hg or DBP >120 mm Hg (Table 3). The available evidence has failed to show benefit of lowering SBP below certain thresholds (ie, 180 or 160 mm Hg).Evidence 6High Quality of Evidence (high confidence that we know true effects of the intervention). He J, Zhang Y, Xu T, et al; CATIS Investigators. Effects of immediate blood pressure reduction on death and major disability in patients with acute ischemic stroke: the CATIS randomized clinical trial. JAMA. 2014 Feb 5;311(5):479-89. doi: 10.1001/jama.2013.282543. PMID: 24240777. ENOS Trial Investigators, Bath PM, Woodhouse L, Scutt P, et al. Efficacy of nitric oxide, with or without continuing antihypertensive treatment, for management of high blood pressure in acute stroke (ENOS): a partial-factorial randomised controlled trial. Lancet. 2015 Feb 14;385(9968):617-28. doi: 10.1016/S0140-6736(14)61121-1. Epub 2014 Oct 21. Erratum in: Lancet. 2015 Feb 14;385(9968):606. PMID: 25465108; PMCID: PMC4343308. Lattanzi S, Cagnetti C, Provinciali L, Silvestrini M. How should we lower blood pressure after cerebral hemorrhage? A systematic review and meta-analysis. Cerebrovasc Dis. 2017;43(5-6):207-213. doi: 10.1159/000462986. Epub 2017 Feb 28. PMID: 28241129. Sandset EC, Anderson CS, Bath PM, et al. European Stroke Organisation (ESO) guidelines on blood pressure management in acute ischaemic stroke and intracerebral haemorrhage. Eur Stroke J. 2021 Jun;6(2):II. doi: 10.1177/23969873211026998. Epub 2021 Jun 18. PMID: 34780579; PMCID: PMC8370067.
c) Patients with ICH: SBP lowering to 140 mm Hg is suggested as it is safe and probably beneficial (Table 4). It is probably safe to rapidly lower the SBP to 140 or less; however, the efficacy is unproven.Evidence 7High Quality of Evidence (high confidence that we know true effects of the intervention). He J, Zhang Y, Xu T, et al; CATIS Investigators. Effects of immediate blood pressure reduction on death and major disability in patients with acute ischemic stroke: the CATIS randomized clinical trial. JAMA. 2014 Feb 5;311(5):479-89. doi: 10.1001/jama.2013.282543. PMID: 24240777. ENOS Trial Investigators, Bath PM, Woodhouse L, Scutt P, et al. Efficacy of nitric oxide, with or without continuing antihypertensive treatment, for management of high blood pressure in acute stroke (ENOS): a partial-factorial randomised controlled trial. Lancet. 2015 Feb 14;385(9968):617-28. doi: 10.1016/S0140-6736(14)61121-1. Epub 2014 Oct 21. Erratum in: Lancet. 2015 Feb 14;385(9968):606. PMID: 25465108; PMCID: PMC4343308. Lattanzi S, Cagnetti C, Provinciali L, Silvestrini M. How should we lower blood pressure after cerebral hemorrhage? A systematic review and meta-analysis. Cerebrovasc Dis. 2017;43(5-6):207-213. doi: 10.1159/000462986. Epub 2017 Feb 28. PMID: 28241129. Sandset EC, Anderson CS, Bath PM, et al. European Stroke Organisation (ESO) guidelines on blood pressure management in acute ischaemic stroke and intracerebral haemorrhage. Eur Stroke J. 2021 Jun;6(2):II. doi: 10.1177/23969873211026998. Epub 2021 Jun 18. PMID: 34780579; PMCID: PMC8370067.
d) Patients with SAH: SBP >160 mm Hg.
e) Patients with elevated blood pressure associated with acute coronary syndrome, aortic dissection, heart failure, renal injury, or markedly reduced coagulability in the course of anticoagulant treatment. Blood pressure targets are based on the condition and urgency of the situation.
2) Choice of agents: Blood pressure is best controlled with IV drugs; examples: Table 2, Table 3, Table 4. Stroke is rarely accompanied by hypotension, which is usually associated with ischemic heart disease or may be a result of dehydration, bleeding (usually from the gastrointestinal tract), or pharmacotherapy. Correct the underlying cause of hypotension, administer fluids, and, if necessary, manage the patient in an ICU setting with vasopressors.
3. Correct any existing fluid and electrolyte disturbances (see Electrolyte, Fluid, and Acid-Base Balance Disorders).
4. Monitor blood glucose levels: Both hyperglycemia and hypoglycemia have adverse effects on the brain in the setting of acute stroke. In patients with hyperglycemia restrict dietary carbohydrates. In patients with glucose levels ≥10 mmol/L consider insulin treatment but with caution, to avoid hypoglycemia. In patients with hypoglycemia (<3.3 mmol/L) administer 25 mL of 50% glucose (dextrose) in a slow IV injection (low tonicity of a 5% glucose solution may cause brain edema).
5. Reduce body temperature if it is >37.5 degrees Celsius. Fever is frequent in the first 48 hours after stroke and is associated with a less favorable prognosis. The source of fever, in particular infection, should be identified. Use acetaminophen (INN paracetamol) (up to 4000 mg/d) and surface cooling. Prophylactic use of acetaminophen (INN paracetamol) is not indicated.Evidence 8Strong recommendation (downsides clearly outweigh benefits; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of the intervention). Den Hertog HM, van der Worp HB, van Gemert HM, et al; PAIS investigators. The Paracetamol (Acetaminophen) In Stroke (PAIS) trial: a multicentre, randomised, placebo-controlled, phase III trial. Lancet Neurol. 2009 May 1;8(5):434-40. doi: 10.1016/S1474-4422(09)70051-1. Epub 2009 Mar 16. PMID: 19297248.
6. Monitor urine output: Urinary incontinence is common after acute stroke, and as many as 20% of patients with stroke have urinary retention. Bladder scanning, in-out catheterization, or both should be ordered. Temporary catheterization may be indicated to monitor urine output and in the case of urinary retention; however, prolonged catheterization should be avoided, if possible, as it is associated with increased risk of infections.
7. Insert a nasogastric tube to provide oral medications otherwise not available IV in patients with dysphagia. Dysphagia is common after stroke; however, early feeding does not change the probability of a good outcome and may be deferred as long as hydration is maintained.
8. Start prophylaxis of venous thromboembolism for immobile patients with low-molecular-weight heparin (LMWH) (eg, enoxaparin 40 mg/d) rather than unfractionated heparin (UFH). Consider pneumatic compression if anticoagulation is contraindicatedEvidence 9Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of the intervention). Sandercock PA, Counsell C, Tseng MC. Low-molecular-weight heparins or heparinoids versus standard unfractionated heparin for acute ischaemic stroke. Cochrane Database Syst Rev. 2008 Jul 16;(3):CD000119. doi: 10.1002/14651858.CD000119.pub3. Review. PMID: 18646059. CLOTS (Clots in Legs Or sTockings after Stroke) Trials Collaboration, Dennis M, Sandercock P, Reid J, Graham C, Forbes J, Murray G. Effectiveness of intermittent pneumatic compression in reduction of risk of deep vein thrombosis in patients who have had a stroke (CLOTS 3): a multicentre randomised controlled trial. Lancet. 2013 Aug 10;382(9891):516-24. doi: 10.1016/S0140-6736(13)61050-8. Epub 2013 May 31. Erratum in: Lancet. 2013 Aug 10;382(9891):506. Lancet. 2013 Sep 21;382(9897):1020. PMID: 23727163. (see Primary Prevention of Venous Thromboembolism).
9. Good nursing care and positioning can reduce the risk of aspiration pneumonia, other infections, and pressure ulcers.
10. Management of increased intracranial pressure or seizures: see Complications, below.
Specific Therapy for Ischemic Stroke
1. Acetylsalicylic acid (ASA): Administer ASA immediately in patients who are not treated with fibrinolysis or 24 hours following fibrinolytic treatment; intracranial hemorrhage must be excluded by brain CT. Start with 160 to 325 mg/d, then reduce the dose to 81 mg/d.Evidence 10Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of the intervention). Antithrombotic Trialists' Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002 Jan 12;324(7329):71-86. Erratum in: BMJ 2002 Jan 19;324(7330):141. PMID: 11786451; PMCID: PMC64503. In patients after a minor ischemic stroke or TIA, add clopidogrel 300 to 600 mg as a loading dose followed by 75 mg/d for ~30 days.Evidence 11Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of the intervention). Hao Q, Tampi M, O'Donnell M, Foroutan F, Siemieniuk RA, Guyatt G. Clopidogrel plus aspirin versus aspirin alone for acute minor ischaemic stroke or high risk transient ischaemic attack: systematic review and meta-analysis. BMJ. 2018 Dec 18;363:k5108. doi: 10.1136/bmj.k5108. PMID: 30563866; PMCID: PMC6298178. Rahman H, Khan SU, Nasir F, Hammad T, Meyer MA, Kaluski E. Optimal Duration of Aspirin Plus Clopidogrel After Ischemic Stroke or Transient Ischemic Attack. Stroke. 2019 Apr;50(4):947-953. doi: 10.1161/STROKEAHA.118.023978. PMID: 30852971. Due to increased risk of bleeding, it is preferred to postpone ASA in patients receiving fibrinolytic treatment (for 24 hours) or undergoing clot retrieval (unless a stent is placed during the procedure), but with that consideration both interventions can be used in a person who has already received ASA.
2. Fibrinolytic treatment: Recombinant tissue plasminogen activator (rtPA) 0.9 mg/kg (10% of the dose in IV injection over 1-2 minutes followed by the remaining 90% of the dose as IV infusion over 1 hour; maximum total dose, 90 mg).Evidence 12Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of the intervention). Wardlaw JM, Murray V, Berge E, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet. 2012 Jun 23;379(9834):2364-72. doi: 10.1016/S0140-6736(12)60738-7. Epub 2012 May 23. Review. PMID: 22632907; PMCID: PMC3386494. Wardlaw JM, Murray V, Berge E, del Zoppo GJ. Thrombolysis for Acute Ischemic Stroke, Update August 2014. Stroke. 2014;45(11):e222-e225. doi:10.1161/STROKEAHA.114.007024. Berge E, Whiteley W, Audebert H, et al. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J. 2021 Mar;6(1):I-LXII. doi: 10.1177/2396987321989865. Epub 2021 Feb 19. PMID: 33817340; PMCID: PMC7995316. Specific points:
1) rtPA should be used within 4.5 hours from the onset of an ischemic stroke that has caused clinically significant and measurable neurologic deficits. Note that the benefits of fibrinolytic therapy decrease as more time elapses from the onset of stroke to the administration of rtPA.
2) Recognizing the increased risk of bleeding, rtPA may be used in patients who are currently treated with ASA or have taken it recently, or in patients in whom subsequent clot retrieval is considered.
3) In patients with wake-up stroke or within 9 hours of last seen well, rtPA may still be indicated based on advanced imaging parameters and in consultation with a neurologist or stroke center.
4) Exclusion criteria: Table 5.
5) Complications: The risk of symptomatic intracranial hemorrhage is increased in patients who receive rtPA (7% vs 1%), as is early death; however, death or dependency at 3 to 6 months is still decreased in patients treated with rtPA. The risk of bleeding increases with the time from the onset of symptoms and with severity of stroke.Evidence 13High Quality of Evidence (high confidence that we know true effects of the intervention). Whiteley WN, Emberson J, Lees KR, et al; Stroke Thrombolysis Trialists' Collaboration. Risk of intracerebral haemorrhage with alteplase after acute ischaemic stroke: a secondary analysis of an individual patient data meta-analysis. Lancet Neurol. 2016 Aug;15(9):925-933. doi: 10.1016/S1474-4422(16)30076-X. Epub 2016 Jun 8. PMID: 27289487.
3. For patients with occlusions of the proximal intracranial vessels (carotid artery, middle cerebral artery, anterior cerebral artery) as seen on CTA and within 6 hours of the onset of ischemic stroke, including those who underwent fibrinolytic therapy, intra-arterial clot extraction with stent retrievers significantly improves the probability of good recovery; consideration should be given to treatment or transfer to an interventional stroke center if feasible.Evidence 14Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of the intervention). Berkhemer OA, Fransen PS, Beumer D, et al; MR CLEAN Investigators. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015 Jan 1;372(1):11-20. doi: 10.1056/NEJMoa1411587. Epub 2014 Dec 17. Erratum in: N Engl J Med. 2015 Jan 22;372(4):394. PMID: 25517348. Goyal M, Demchuk AM, Menon BK, et al; ESCAPE Trial Investigators. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015 Mar 12;372(11):1019-30. doi: 10.1056/NEJMoa1414905. Epub 2015 Feb 11. PMID: 25671798. Campbell BC, Mitchell PJ, Kleinig TJ, et al; EXTEND-IA Investigators. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015 Mar 12;372(11):1009-18. doi: 10.1056/NEJMoa1414792. Epub 2015 Feb 11. PMID: 25671797. Saver JL, Goyal M, Bonafe A, et al; SWIFT PRIME Investigators. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015 Jun 11;372(24):2285-95. doi: 10.1056/NEJMoa1415061. Epub 2015 Apr 17. PMID: 25882376. Goyal M, Menon BK, van Zwam WH, et al; HERMES collaborators. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016 Apr 23;387(10029):1723-31. doi: 10.1016/S0140-6736(16)00163-X. Epub 2016 Feb 18. PubMed PMID: 26898852. Jovin TG, Nogueira RG, DAWN Investigators. Thrombectomy 6 to 24 hours after stroke. N Engl J Med. 2018 Mar 22;378(12):1161-2.Jovin TG, Nogueira RG; DAWN Investigators. Thrombectomy 6 to 24 Hours after Stroke. N Engl J Med. 2018 Mar 22;378(12):1161-1162. doi: 10.1056/NEJMc1801530. PubMed PMID: 29562149. Albers GW, Marks MP, Kemp S, et al; DEFUSE 3 Investigators. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N Engl J Med. 2018 Feb 22;378(8):708-718. doi: 10.1056/NEJMoa1713973. Epub 2018 Jan 24. PubMed PMID: 29364767; PubMed Central PMCID: PMC6590673. Patients with occlusion of the proximal intracranial vessels as seen on CTA and within 6 to 24 hours of the onset of ischemic stroke may be candidates for intra-arterial clot extraction if imaging with CT perfusion identifies a significant mismatch between infarcted and potentially salvageable brain tissue.Evidence 15Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of the intervention). Berkhemer OA, Fransen PS, Beumer D, et al; MR CLEAN Investigators. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015 Jan 1;372(1):11-20. doi: 10.1056/NEJMoa1411587. Epub 2014 Dec 17. Erratum in: N Engl J Med. 2015 Jan 22;372(4):394. PMID: 25517348. Goyal M, Demchuk AM, Menon BK, et al; ESCAPE Trial Investigators. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015 Mar 12;372(11):1019-30. doi: 10.1056/NEJMoa1414905. Epub 2015 Feb 11. PMID: 25671798. Campbell BC, Mitchell PJ, Kleinig TJ, et al; EXTEND-IA Investigators. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015 Mar 12;372(11):1009-18. doi: 10.1056/NEJMoa1414792. Epub 2015 Feb 11. PMID: 25671797. Saver JL, Goyal M, Bonafe A, et al; SWIFT PRIME Investigators. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015 Jun 11;372(24):2285-95. doi: 10.1056/NEJMoa1415061. Epub 2015 Apr 17. PMID: 25882376. Goyal M, Menon BK, van Zwam WH, et al; HERMES collaborators. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016 Apr 23;387(10029):1723-31. doi: 10.1016/S0140-6736(16)00163-X. Epub 2016 Feb 18. PubMed PMID: 26898852. Jovin TG, Nogueira RG, DAWN Investigators. Thrombectomy 6 to 24 hours after stroke. N Engl J Med. 2018 Mar 22;378(12):1161-2.Jovin TG, Nogueira RG; DAWN Investigators. Thrombectomy 6 to 24 Hours after Stroke. N Engl J Med. 2018 Mar 22;378(12):1161-1162. doi: 10.1056/NEJMc1801530. PubMed PMID: 29562149. Albers GW, Marks MP, Kemp S, et al; DEFUSE 3 Investigators. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N Engl J Med. 2018 Feb 22;378(8):708-718. doi: 10.1056/NEJMoa1713973. Epub 2018 Jan 24. PubMed PMID: 29364767; PubMed Central PMCID: PMC6590673.
4. Heparin:
1) Ischemic stroke: Administration of UFH or LMWH at therapeutic doses (the majority of immobilized patients require the use of heparin at prophylactic doses) is indicated only in exceptional cases: possibly in patients with stroke caused by cardioembolism who are at high risk of a second embolism as well as in patients with arterial dissection. However, even in these circumstances there is no generally accepted evidence that the benefit from treatment outweighs the risk of increased bleeding.
2) Cerebral venous thrombosis: Start with heparin (UFH or LMWH) at therapeutic doses (Table 2 in Deep Vein Thrombosis), then continue treatment with a vitamin K antagonist (VKA) (warfarin) for 3 to 6 months (INR, 2.0-3.0). Evidence for the use of direct oral anticoagulants (DOACs) is limited. Repeat venous imaging should be used to guide discontinuation of anticoagulation.Evidence 16Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due to the low number of events. Coutinho J, de Bruijn SF, Deveber G, Stam J. Anticoagulation for cerebral venous sinus thrombosis. Cochrane Database Syst Rev. 2011 Aug 10;(8):CD002005. doi: 10.1002/14651858.CD002005.pub2. Review. PMID: 21833941. Saposnik G, Barinagarrementeria F, Brown RD Jr, et al; American Heart Association Stroke Council and the Council on Epidemiology and Prevention. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011 Apr;42(4):1158-92. doi: 10.1161/STR.0b013e31820a8364. Epub 2011 Feb 3. Review. PMID: 21293023. Ferro JM, Bousser MG, Canhão P, et al. European Stroke Organization guideline for the diagnosis and treatment of cerebral venous thrombosis - Endorsed by the European Academy of Neurology. Eur Stroke J. 2017 Sep;2(3):195-221. doi: 10.1177/2396987317719364. Epub 2017 Jul 21. PMID: 31008314; PMCID: PMC6454824.
3) Contraindications: Extensive ischemic infarction (eg, involving >50% of the area supplied by the middle cerebral artery), uncontrolled hypertension, hemorrhagic transformation of ischemic infarction, and stroke complicating infective bacterial endocarditis.
Specific Therapy for ICH and SAH
SAH requires surgical clipping of the ruptured vessel and/or endovascular embolization using coils inserted into the aneurysm. Neurosurgical consultation should be obtained in all patients with SAH.
Surgical evacuation of an ICH is generally not recommended, except for selected patients with cerebellar hematoma to prevent fatal brain stem compression and in patients with a large spontaneous superficial ICH with impending herniation. Neurosurgical consultation is recommended for most patients with ICH.
Rehabilitation plays a crucial role in the resolution of neurologic deficits caused by stroke. The goals of rehabilitation are to prevent complications, minimize loss of function (impairment), and maximize activity and participation (disability and handicap). Patients should be admitted to and managed in a rehabilitation stroke unit by a multidisciplinary stroke team that includes the core disciplines of physiotherapy, occupational therapy, and speech language therapy.
Complications Top
Progressive stroke, brain edema, hemorrhagic transformation, and recurrent infarction are the neurologic causes of clinical deterioration.
1. Increased intracranial pressure and brain edema, which develops as early as 24 to 48 hours of stroke, after vascular reperfusion, usually peaks after 3 to 5 days. Increasing edema is a frequent cause of worsening neurologic deficits (affecting about 10% of patients); it may lead to cerebral herniation and death.
Treatment:
1) Elevate the head of the bed at 20 to 30 degrees.
2) Provide a comfortable environment and prevent secondary complications (eg, adequate pain management, appropriate body positioning, shoulder protection in hemiplegic patients).
3) Prevent hypoxemia and maintain a normal body temperature.
4) Short-term hyperventilation (unproven efficacy for improving outcomes) may be used (provided cerebral perfusion is good). Lowering partial pressure of carbon dioxide (PaCO2) by 5 to 10 mm Hg may decrease intracranial pressure by 25% to 30%.
5) Pharmacologic treatment (unproven efficacy) might be considered only in the case of massive cerebral edema: IV mannitol 0.25 to 0.5 g/kg over 20 minutes (this may be repeated every 6 hours) or hypertonic saline. Glucocorticoids have not been shown to be effective.
6) In younger patients (<60 years) with massive hemispheric cerebral infarction (>2/3 of the middle cerebral artery territory), early decompressive craniectomy decreases mortality from ~80% to 30% but with potentially severe residual disability; the decision should be guided by patient values and preferences.Evidence 17Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). High Quality of Evidence (high confidence that we know true effects of the intervention). Vahedi K, Hofmeijer J, Juettler E, et al; DECIMAL, DESTINY, and HAMLET investigators. Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol. 2007 Mar;6(3):215-22. PMID: 17303527. In older individuals (>60 years) decompressive craniectomy increases survival but only at the expense of severe disability.Evidence 18Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due indirectness (difficulties in interpretation of severe disability). Jüttler E, Unterberg A, Woitzik J, et al; DESTINY II Investigators. Hemicraniectomy in older patients with extensive middle-cerebral-artery stroke. N Engl J Med. 2014 Mar 20;370(12):1091-100. doi: 10.1056/NEJMoa1311367. PMID: 24645942.
7) Cerebellar infarction or hemorrhage is particularly dangerous and prone to life-ending herniation. Close monitoring and early neurosurgical consultation should be obtained. Patients may require early craniectomy or ventricular drainage.
2. Epileptic seizures, which are usually partial or secondarily generalized partial seizures (rarely status epilepticus), may occur early or later after stroke in 5% to 15% of patients; recurrence is not uncommon. Treatment of seizures may require immediate control with IV lorazepam 2 to 4 mg (maximum, 8 mg), followed by phenytoin 15 to 20 mg/kg by IV infusion (maximum rate, 50 mg/min). Long-term treatment is preferred with either lamotrigine, levetiracetam, carbamazepine, or gabapentin; drug choice is dependent on other patient factors.
3. Venous thromboembolism and pulmonary embolism: Prevention and treatment: see Deep Vein Thrombosis; see Pulmonary Embolism; see Primary Prevention of Venous Thromboembolism.
4. Infections:
1) Urinary tract infections occur in up to 15% of patients within the first 2 weeks of stroke and may be prevented by maintaining adequate fluid volume and avoiding unnecessary catheterization.
2) Respiratory infections most often occur in the context of dysphagia and more severe strokes in up to 7% of patients and are associated with increased mortality and poor functional outcomes. Assessment and monitoring for dysphagia, early mobilization, and early treatment of infection can improve outcomes.
5. Urinary and fecal incontinence: Urinary incontinence is present in up to 50% of patients early after stroke and persists in up to a third of patients 1 year later. Avoid aggravating factors (eg, diuretics), treat urinary tract infections with appropriate antibiotics, and monitor for urinary retention. Constipation may occur in up to 50% of patients within the first month of stroke. Proper hydration, monitoring, and mobilization may prevent complications.
6. Pressure ulcers: Due to immobility and incontinence, patients with stroke are at high risk of skin breakdown. Monitoring, skin protection, and positioning are the key preventive measures.
7. Spasticity is a velocity-dependent increase in tone that occurs after stroke. It can cause pain and decrease in function and interfere with recovery. Positioning, stretching, a range of motion exercises, and other physiotherapeutic interventions are the mainstay of treatment. Antispasmodic medication (avoiding benzodiazepines) and botulinum toxin injections are part of a comprehensive spasticity-management program.
8. Shoulder pain syndrome: A painful shoulder has many causes, including trauma, bursitis/tendonitis/capsulitis, rotator cuff tears, spasticity, and complex regional pain syndrome 1 or 2. Appropriate shoulder protection is necessary as a prophylactic measure; start physiotherapy and use analgesics if needed (glucocorticoid injections are not recommended unless there is a specific indication). Advise the patient and the caregiver how to get up from the bed without straining the affected shoulder and how to support the affected limb, especially if the limb is flaccid. Treatment of spasticity and restoration of a normal range of motion require specialized procedures (avoid exercises using weights suspended over the head).
9. Falls: Falls may occur in 15% to 65% of stroke patients. Strategies for the prevention of falls are necessary.
10. Malnutrition: Patients unable to eat on their own or with dysphagia are at risk for dehydration and malnutrition. Maintain adequate hydration and nutrition; introduce nasogastric or percutaneous endoscopic gastrostomy (PEG) feedings if necessary and desired. Note that early nasogastric feeding is not associated with better outcomes.Evidence 19Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of the intervention). Dennis MS, Lewis SC, Warlow C; FOOD Trial Collaboration. Effect of timing and method of enteral tube feeding for dysphagic stroke patients (FOOD): a multicentre randomised controlled trial. Lancet. 2005 Feb 26-Mar 4;365(9461):764-72. PMID: 15733717.
11. Depression (affects ~30% of patients at different periods following stroke): Treatment with selective serotonin reuptake inhibitors (SSRIs) should be considered. The use of SSRIs improves depression but does not improve functional outcomes.Evidence 20Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of the intervention). FOCUS Trial Collaboration. Effects of fluoxetine on functional outcomes after acute stroke (FOCUS): a pragmatic, double-blind, randomised, controlled trial. Lancet. 2019 Jan 19;393(10168):265-274. doi: 10.1016/S0140-6736(18)32823-X. Epub 2018 Dec 5. PMID: 30528472; PMCID: PMC6336936.
12. Delirium is common after stroke and affects up to a quarter of patients. An underlying cause aside from stroke should be sought and treated if found.
Prevention Top
1. Management of risk factors:
1) Hypertension: Start treatment with an angiotensin-converting enzyme inhibitor (ACEI) in stable patients after a few days (on average 7) targeting an SBP <140 mm Hg and a DBP <90 mm HgEvidence 21Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of the intervention). PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001 Sep 29;358(9287):1033-41. Erratum in: Lancet 2001 Nov 3;358(9292):1556. Lancet 2002 Jun 15;359(9323):2120. PMID: 11589932. Liu L, Wang Z, Gong L, et al. Blood pressure reduction for the secondary prevention of stroke: a Chinese trial and a systematic review of the literature. Hypertens Res. 2009 Nov;32(11):1032-40. doi: 10.1038/hr.2009.139. Epub 2009 Oct 2. Review. PMID: 19798097.; for patients with LACI a treatment target of an SBP <130 mm Hg is indicated.Evidence 22Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of the intervention). SPS3 Study Group, Benavente OR, Coffey CS, Conwit R, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013 Aug 10;382(9891):507-15. doi: 10.1016/S0140-6736(13)60852-1. Epub 2013 May 29. Erratum in: Lancet. 2013 Aug 10;382(9891):506. Coffey, C S [aded]. PMID: 23726159; PMCID: PMC3979302. Initiate treatment with an ACEI (perindopril or ramipril) and/or diuretic (eg, indapamide).
2) Glucose intolerance and diabetes mellitus: These are best assessed by measuring glycated hemoglobin (HbA1c). Follow treatment guidelines for nonstroke and mixed patient populations.
3) Hypercholesterolemia: High-dose statin treatment (eg, atorvastatin 80 mg/d) should be initiated in all patients with an ischemic stroke or TIA presumed to be of atherosclerotic origin and then hypercholesterolemia should be treated to achieve a low-density lipoprotein (LDL) cholesterol target of <1.8 mmol/L.Evidence 23Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of the intervention). Amarenco P, Bogousslavsky J, Callahan A 3rd, et al; Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006 Aug 10;355(6):549-59. PMID: 16899775. O'Regan C, Wu P, Arora P, Perri D, Mills EJ. Statin therapy in stroke prevention: a meta-analysis involving 121,000 patients. Am J Med. 2008 Jan;121(1):24-33. doi: 10.1016/j.amjmed.2007.06.033. Review. PMID: 18187070. Amarenco P, Kim JS, Labreuche J, et al. A comparison of two LDL cholesterol targets after ischemic stroke. N Engl J Med. 2020 Jan 2;382(1):9. doi: 10.1056/NEJMoa1910355. Epub 2019 Nov 18. PMID: 31738483.
4) Cessation of smoking: Continued smoking is associated with stroke recurrence. Behavioral therapy and pharmacotherapy are effective in treating tobacco dependence.
5) Screening for obesity, weight loss measures: Although screening is recommended, weight loss is of uncertain benefit.
6) Regular physical activity: Those patients who can and have no other contraindications should participate in moderate to vigorous intensity exercise 3 to 4 times per week for 40 minutes.
7) Obstructive sleep apnea (OSA): There is a high prevalence of OSA in patients with TIA and stroke. Although treatment with continuous positive airway pressure (CPAP) reduces sleepiness and other symptoms of OSA, it does not prevent cardiovascular events in patients with moderate to severe OSA.Evidence 24Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due to indirectness. McEvoy RD, Antic NA, Heeley E, et al; SAVE Investigators and Coordinators. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. N Engl J Med. 2016 Sep 8;375(10):919-31. doi: 10.1056/NEJMoa1606599. Epub 2016 Aug 28. PMID: 27571048.
8) Alcohol: Light to moderate amounts of alcohol may have protective effects (2 drinks per day for men, 1 drink per day for women). Heavier use is associated with an increased risk of ischemic and hemorrhagic stroke.
2. Antithrombotic treatment: Treatment is dependent on the presumed cause of stroke.
1) Noncardioembolic sources of TIA or stroke (extracranial atherosclerotic arterial disease, intracranial atherosclerotic arterial disease, lacunar disease): Use antiplatelet treatment, not anticoagulation.Evidence 25Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of the intervention). Antithrombotic Trialists' Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002 Jan 12;324(7329):71-86. Erratum in: BMJ 2002 Jan 19;324(7330):141. PMID: 11786451; PMCID: PMC64503. Options include ASA 81 to 325 mg/d, clopidogrel 75 mg/d, or ASA 25 mg + extended-release dipyridamole bid. The combination therapy (ASA + dipyridamole) and clopidogrel have similar efficacy. A combination of ASA 81 mg/d and clopidogrel 75 mg/d may be used for the first 30 days after TIA or stroke or 90 days in patients with intracranial disease; long-term treatment without other indications is associated with increased risk of bleeding and should not be used. Triple antiplatelet therapy (ASA + clopidogrel + dipyridamole) is associated with increased risk of major bleeding without benefit over clopidogrel alone or the combination of ASA and dipyridamole.Evidence 26Strong recommendation (downsides clearly outweigh benefits; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of the intervention). Wang Y, Wang Y, Zhao X, et al; CHANCE Investigators. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013 Jul 4;369(1):11-9. doi: 10.1056/NEJMoa1215340. Epub 2013 Jun 26. PMID: 23803136. Derdeyn CP, Chimowitz MI, Lynn MJ, et al; Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis Trial Investigators. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial. Lancet. 2014 Jan 25;383(9914):333-41. doi: 10.1016/S0140-6736(13)62038-3. Epub 2013 Oct 26. PMID: 24168957; PMCID: PMC3971471. Diener HC, Bogousslavsky J, Brass LM, et al; MATCH investigators. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004 Jul 24-30;364(9431):331-7. PMID: 15276392. Bath PM, Woodhouse LJ, Appleton JP, et al; TARDIS Investigators. Antiplatelet therapy with aspirin, clopidogrel, and dipyridamole versus clopidogrel alone or aspirin and dipyridamole in patients with acute cerebral ischaemia (TARDIS): a randomised, open-label, phase 3 superiority trial. Lancet. 2018 Mar 3;391(10123):850-859. doi: 10.1016/S0140-6736(17)32849-0. Epub 2017 Dec 20. PMID: 29274727; PMCID: PMC5854459.
2) Cardioembolic sources of TIA or stroke (atrial fibrillation, cardiomyopathy, left ventricular thrombus, valvular heart disease [native, bioprosthetic, mechanical]): In patients with atrial fibrillation long-term anticoagulation is indicated to prevent recurrent stroke.Evidence 27Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of the intervention). Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007 Jun 19;146(12):857-67. PMID: 17577005. Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009 Sep 17;361(12):1139-51. doi: 10.1056/NEJMoa0905561. Epub 2009 Aug 30. Erratum in: N Engl J Med. 2010 Nov 4;363(19):1877. PMID: 19717844. Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011 Sep 8;365(10):883-91. doi:10.1056/NEJMoa1009638. Epub 2011 Aug 10. PMID: 21830957. Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011 Sep 15;365(11):981-92. doi: 10.1056/NEJMoa1107039. Epub 2011 Aug 27. PMID: 21870978. Use a VKA or non-VKA oral anticoagulants (DOACs; apixaban, dabigatran, rivaroxaban). The target INR for a VKA is 2.5 (range, 2.0-3.0). DOACs are chosen based on the patient’s preference, tolerability, drug interactions, renal dysfunction, and time in range for VKA treatment. ASA 81 mg/d is indicated if anticoagulation is not possible; ASA 81 mg/d with clopidogrel 75 mg/d may also be considered if anticoagulation is not possible.Evidence 28Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). High Quality of Evidence (high confidence that we know true effects of the intervention). ACTIVE Investigators, Connolly SJ, Pogue J, Hart RG, et al. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med. 2009 May 14;360(20):2066-78. doi: 10.1056/NEJMoa0901301. Epub 2009 Mar 31. PMID: 19336502. Single antiplatelet therapy may be added to anticoagulation in patients with atrial fibrillation and acute coronary syndrome or a coronary stent; dual antiplatelet therapy plus anticoagulation is associated with increased risk of bleeding without a decrease in the rate of thromboembolic events.Evidence 29Strong recommendation (downsides clearly outweigh benefits; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of the intervention). Cavallari I, Patti G. Meta-Analysis Comparing the Safety and Efficacy of Dual Versus Triple Antithrombotic Therapy in Patients With Atrial Fibrillation Undergoing Percutaneous Coronary Intervention. Am J Cardiol. 2018 Mar 15;121(6):718-724. doi: 10.1016/j.amjcard.2017.12.014. Epub 2017 Dec 25. PMID: 29373105. Anticoagulation with a VKA is indicated for a left ventricular thrombus; the target INR is 2.5 (range, 2.0-3.0).
3) Long-term anticoagulation with a VKA is recommended for the prevention of stroke in patients with mechanical heart valves; the intensity of anticoagulation varies by the valve position (aortic: INR, 2.0-3.0; mitral: INR, 2.5-3.5). The addition of ASA 81 to 100 mg/d in patients at a lower risk of bleeding decreases the risk of embolism. DOACs should not be used in patients with mechanical valves as these agents increase the risk of embolism and bleeding.Evidence 30Strong recommendation (downsides clearly outweigh benefits; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of the intervention). Eikelboom JW, Connolly SJ, Brueckmann M, et al; RE-ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013 Sep 26;369(13):1206-14. doi: 10.1056/NEJMoa1300615. Epub 2013 Aug 31. PMID: 23991661. The risk of embolism and recurrent embolism is lower with bioprosthetic valves, and long-term anticoagulation over antiplatelet treatment may not be indicated.
4) Patients with dilated or restrictive cardiomyopathy (left ventricular ejection fraction ≤35%) with sinus rhythm and without a thrombus have a relatively low risk of recurrent embolism. Antiplatelet treatment rather than a VKA or DOAC may be reasonable.Evidence 31Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of the intervention). Homma S, Thompson JL, Pullicino PM, et al; WARCEF Investigators. Warfarin and aspirin in patients with heart failure and sinus rhythm. N Engl J Med. 2012 May 17;366(20):1859-69. doi: 10.1056/NEJMoa1202299. Epub 2012 May 2. PMID: 22551105; PMCID: PMC3723382. Zannad F, Anker SD, Byra WM, et al; COMMANDER HF Investigators. Rivaroxaban in Patients with Heart Failure, Sinus Rhythm, and Coronary Disease. N Engl J Med. 2018 Oct 4;379(14):1332-1342. doi: 10.1056/NEJMoa1808848. Epub 2018 Aug 27. PMID: 30146935.
5) Other, less common sources of stroke are arterial dissection, aortic arch disease, PFO, and cerebral vein and sinus thrombosis. One recent randomized trial compared antiplatelet treatment with anticoagulation treatment in cervical (carotid or vertebral) arterial dissection and found no difference between these treatments; however, the study was associated with major imprecision that precluded a clear recommendation.Evidence 32Low Quality of Evidence (low confidence that we know true effects of the intervention). Quality of Evidence lowered due to the small number of events. CADISS trial investigators, Markus HS, Hayter E, Levi C, Feldman A, Venables G, Norris J. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trial. Lancet Neurol. 2015 Apr;14(4):361-7. doi: 10.1016/S1474-4422(15)70018-9. Epub 2015 Feb 12. Erratum in: Lancet Neurol. 2015 Jun;14(6):566. PMID: 25684164. Debette S, Mazighi M, Bijlenga P, et al. ESO guideline for the management of extracranial and intracranial artery dissection. Eur Stroke J. 2021 Sep;6(3):XXXIX-LXXXVIII. doi: 10.1177/23969873211046475. Epub 2021 Oct 13. PMID: 34746432; PMCID: PMC8564160. Aortic arch disease has been associated with increased risk of stroke, and in such cases antiplatelet therapy with statin therapy is suggested over anticoagulation.Evidence 33Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). Low Quality of Evidence (low confidence that we know true effects of the intervention). Quality of Evidence lowered due to the small number of events. Amarenco P, Davis S, Jones EF, et al; Aortic Arch Related Cerebral Hazard Trial Investigators. Clopidogrel plus aspirin versus warfarin in patients with stroke and aortic arch plaques. Stroke. 2014 May;45(5):1248-57. doi: 10.1161/STROKEAHA.113.004251. Epub 2014 Apr 3. PMID: 24699050. A PFO can be a source of embolism with or without paradoxical embolism but is often an “innocent bystander” (found in 23% of the general population); antiplatelet and anticoagulant therapy seem equally effective in preventing recurrence of stroke. PFO closure is associated with a small increased risk of periprocedural new-onset atrial fibrillation but with a long-term reduction in recurrent stroke in carefully selected patients <60 years of age with confirmed stroke, TIA, or systemic embolism and high probability of PFO-associated event.Evidence 34Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). High Quality of Evidence (high confidence that we know true effects of the intervention). Nasir UB, Qureshi WT, Jogu H, et al. Updated meta-analysis of closure of patent foramen ovale versus medical therapy after cryptogenic stroke. Cardiovasc Revasc Med. 2019 Mar;20(3):187-193. doi: 10.1016/j.carrev.2018.06.001. Epub 2018 Jun 13. PMID: 30905408. Pristipino C, Sievert H, D'Ascenzo F, et al; Evidence Synthesis Team; Eapci Scientific Documents and Initiatives Committee; International Experts. European position paper on the management of patients with patent foramen ovale. General approach and left circulation thromboembolism. Eur Heart J. 2018 Oct 25. doi: 10.1093/eurheartj/ehy649. [Epub ahead of print] PMID: 30358849. Cerebral vein and sinus thrombosis is treated acutely with therapeutic doses of LMWH despite the possibility of hemorrhagic conversion of venous infarctions.Evidence 35Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due to the small number of events (imprecision). Coutinho J, de Bruijn SF, Deveber G, Stam J. Anticoagulation for cerebral venous sinus thrombosis. Cochrane Database Syst Rev. 2011 Aug 10;(8):CD002005. doi: 10.1002/14651858.CD002005.pub2. Review. PMID: 21833941. Ferro JM, Bousser MG, Canhão P, et al. European Stroke Organization guideline for the diagnosis and treatment of cerebral venous thrombosis - Endorsed by the European Academy of Neurology. Eur Stroke J. 2017 Sep;2(3):195-221. doi: 10.1177/2396987317719364. Epub 2017 Jul 21. PMID: 31008314; PMCID: PMC6454824. Long-term anticoagulation of this condition (3 months vs longer) is based on indirect evidence from other venous thrombosis territories and depends on the individual patient’s circumstances (presence of precipitating factors or thrombophilias).
In the absence of carotid artery stenosis warranting revascularization or a major-risk cardioembolic source requiring anticoagulation (such as atrial fibrillation or a left ventricular thrombus), the 3 most important mainstays of secondary ischemic stroke risk reduction are blood pressure control, antiplatelet therapy, and high-dose statins.
3. Invasive treatment of carotid artery stenosis: see Carotid and Vertebral Artery Disease.
Tables and FiguresTop
|
1a. LOC |
0 |
Alert | |
|
1 |
Drowsy | ||
|
2 |
Stuporous | ||
|
3 |
Comatose | ||
|
1b. LOC questions |
0 |
Answers both questions correctly | |
|
1 |
Answers 1 question correctly | ||
|
2 |
Answers neither question correctly | ||
|
1c. LOC commands |
0 |
Performs both tasks correctly | |
|
1 |
Performs 1 task correctly | ||
|
2 |
Performs neither task correctly | ||
|
2. Best gaze |
0 |
Normal | |
|
1 |
Partial gaze palsy | ||
|
2 |
Forced deviation | ||
|
3. Visual fields |
0 |
No visual loss (or in coma) | |
|
1 |
Partial hemianopia | ||
|
2 |
Complete hemianopia | ||
|
3 |
Bilateral hemianopia | ||
|
4. Facial palsy |
0 |
Normal | |
|
1 |
Minor | ||
|
2 |
Partial | ||
|
3 |
Complete | ||
|
5. Best motor: right arm |
0 |
No drift | |
|
1 |
Drift | ||
|
2 |
Some effort against gravity | ||
|
3 |
No effort against gravity | ||
|
4 |
No movement | ||
|
6. Best motor: left arm |
0 |
No drift | |
|
1 |
Drift | ||
|
2 |
Some effort against gravity | ||
|
3 |
No effort against gravity | ||
|
4 |
No movement | ||
|
7. Best motor: right leg |
0 |
No drift | |
|
1 |
Drift | ||
|
2 |
Some effort against gravity | ||
|
3 |
No effort against gravity | ||
|
4 |
No movement | ||
|
8. Best motor: left leg |
0 |
No drift | |
|
1 |
Drift | ||
|
2 |
Some effort against gravity | ||
|
3 |
No effort against gravity | ||
|
4 |
No movement | ||
|
9. Limb ataxia |
0 |
Absent (or in coma) | |
|
1 |
Present in 1 limb | ||
|
2 |
Present in ≥2 limbs | ||
|
10. Sensory |
0 |
Normal | |
|
1 |
Partial loss | ||
|
2 |
Dense loss (or in coma) | ||
|
11. Best language |
0 |
No dysphasia | |
|
1 |
Mild | ||
|
2 |
Severe dysphasia | ||
|
3 |
Mute | ||
|
12. Dysarthria |
0 |
Normal articulation | |
|
1 |
Mild to moderate dysarthria | ||
|
2 |
Unintelligible or worse | ||
|
13. Neglect |
0 |
No neglect (or in coma) | |
|
1 |
Partial neglect | ||
|
2 |
Complete neglect | ||
|
NIHSS total score |
|||
|
NIHSS scoring for aphasic and comatose patients: 1b. LOC questions: Aphasic and stuporous patients unable to state age or month = 2 points. 2. Best gaze: Conjugate gaze deviation overcome by voluntary or reflex movement = 1 point. Isolated eye nerve palsy = 1 point. 3. Visual fields: Visual field clear cut asymmetry = 1 point. Total blindness = 2 points. 4. Facial palsy: Facial asymmetry can be assessed in response to noxious stimuli. 5-8. Motor testing: Motor testing can be pantomimed for aphasic patients. 9. Limb ataxia: If cannot be demonstrated = 0 points. 10. Sensory: Asymmetry = 1 point. Bilateral sensory loss = 2 points. Patient in coma and with no response to pain = 2 points. 11. Best language: Patient in coma = 3 points. 12. Dysarthria: Scored only if audible speech is heard. 13. Neglect: Present if obviously more than blindness or sensory loss. | |||
|
LOC, level of consciousness; NIHSS, National Institute of Health Stroke Scale. | |||
|
Before fibrinolytic treatment | |
|
Do not administer rtPA if BP cannot be maintained ≤185/110 mm Hg | |
|
Patient eligible for rtPA administration except for SBP >185 mm Hg and/or DBP >110 mm Hg |
– IV labetalol 10-20 mg over 1-2 min, may be repeated once or – IV nicardipine 5 mg/h, titrate up by 2.5 mg/h every 5-15 min (max rate, 15 mg/h). When target BP is achieved, adjust to maintain appropriate BP range or – IV clevidipine 1-2 mg titrated by doubling the dose every 2-5 min until target is reached (max, 21 mg/h) or – Other agents (eg, hydralazine, enalaprilat) may be considered |
|
During or after fibrinolytic treatment | |
|
– Maintain BP ≤180/105 mm Hg (use IV labetalol 10 mg followed by infusion 2-8 mg/h, nicardipine (max, 15 mg/h), or clevidipine (max, 21 mg/h) – Monitor BP every 15 min for 2 h following the start of rtPA administration, then every 30 min for 6 h, and then every hour for 16 h | |
|
BP cannot be controlled with labetalol or nicardipine or DBP >140 mm Hg |
– Admission to ICU – IV sodium nitroprusside 0.5 microg/kg/min |
|
BP, blood pressure; DBP, diastolic blood pressure; ICU, intensive care unit; rtPA, recombinant tissue plasminogen activator; SBP, systolic blood pressure. | |
|
Blood pressure |
Managementa |
|
SBP <220 mm Hg and DBP <120 mm Hg |
Do not use antihypertensive agents. Hold 50% of previously used beta-blockers, hold other antihypertensive medications. Consider antihypertensive treatment in patients with severe heart failure, aortic dissection, or symptoms of hypertensive encephalopathy (choice of agents: see table 12.4-2) |
|
SBP >220 mm Hg or DBP of 120-140 mm Hg |
Benefit of lowering is uncertain but suggested. Lowering BP by 15% within the first 24 h and use of medications (see table 12.4-2) is reasonable (IV labetalol, nicardipine, clevidipine, hydralazine, enalaprilat [0.625-1.25 mg every 6 h]) |
|
DBP >140 mm Hg |
– ICU admission – IV sodium nitroprusside 0.5 microg/kg/min |
|
a Target BP reduction is 10%-15%. Continuous BP monitoring is necessary. Onset, duration of action, and adverse effects of the drugs: see table 3.9-2. | |
|
BP, blood pressure; DBP, diastolic blood pressure; ICU, intensive care unit; SBP, systolic blood pressure. | |
|
BP |
Management |
|
Systolic BP at presentation >140-150 mm Hg, normal ICP; maintain BP ≤140/80 mm Hg |
IV nicardipine 5 mg/h, titrate up by 2.5 mg/h every 5-15 min (max rate, 15 mg/h); IV labetalol, enalapril, or nitroprusside could be used |
|
If ICP is elevated, maintain cerebral perfusion pressure |
ICU admission with ICP monitoring |
|
BP, blood pressure; ICP, intracranial pressure; ICU, intensive care unit. | |
|
Exclusion criteria for thrombolytic therapy with rtPA for acute stroke order set Goal: Door-to-needle time: 60 minutes | ||
|
Absolute exclusion criteria: All answers must be “NO” | ||
|
Onset of symptoms or the “last seen normal” is >4.5 h |
Yes |
No |
|
CT evidence of cerebral hemorrhage |
Yes |
No |
|
Clinical presentation consistent with subarachnoid hemorrhage even if CT scan normal |
Yes |
No |
|
Blood pressure >185/110 mm Hg and not treatable |
Yes |
No |
|
Blood glucose level <2.7 mmol/L (48.6 mg/dL) |
Yes |
No |
|
Significant head trauma, brain surgery, or spinal surgery within 3 months |
Yes |
No |
|
History of intracranial hemorrhage (in previous 6 months) |
Yes |
No |
|
Recent minor stroke (1 month) or moderate to severe stroke (3 months) |
Yes |
No |
|
Active internal, gastrointestinal, or urinary bleeding within 21 days |
Yes |
No |
|
Arterial puncture at a noncompressible site in previous 7 days |
Yes |
No |
|
Platelet count <100×109/L |
Yes |
No |
|
IV heparin received within 48 h, resulting in abnormally elevated aPTT >40 s |
Yes |
No |
|
Low-molecular-weight heparin at full anticoagulant levels |
Yes |
No |
|
Warfarin use with INR >1.7 |
Yes |
No |
|
Direct oral anticoagulants (rivaroxaban, dabigatran, apixaban) taken within previous 48 ha |
Yes |
No |
|
Relative contraindications: Consider eligibility on an individual basis based on benefits and risks | ||
|
Rapidly improving neurologic signs or NIHSS <4 |
Yes |
No |
|
History of arteriovenous malformation or aneurysm |
Yes |
No |
|
Profound stroke with obtundation, fixed eye deviation, and complete hemiplegia, or NIHSS >24 |
Yes |
No |
|
Acute cerebral infarct with ASPECTS ≤5 |
Yes |
No |
|
Recent large myocardial infarction or pericarditis within 3 months |
Yes |
No |
|
Blood glucose level >22.2 mmol/L (399.6 mg/dL) |
Yes |
No |
|
Recent major surgery or trauma (cardiac, thoracic, abdominal, orthopedic) within 14 days |
Yes |
No |
|
History of bleeding diathesis or liver failure |
Yes |
No |
|
Seizure at onset of stroke with residual postictal neurologic deficits |
Yes |
No |
|
Pregnancy |
Yes |
No |
|
Age <18 years |
Yes |
No |
|
Summary eligibility assessment: All answers must be checked | ||
|
1. The patient meets inclusion criteria: acute ischemic stroke symptom and symptom onset <4.5 h. |
Yes |
No |
|
2. The patient does not have any of the absolute exclusion criteria. |
Yes |
No |
|
3. The patient may have one or more of the relative contraindications, but potential benefits of alteplase exceed potential risks. |
Yes |
No |
|
Alteplase (rtPA) to be given (YES or NO): |
Yes |
No |
|
a Some experts consider the reversal of dabigatran with idarucizumab and the use of rtPA in selected patients.Evidence 36Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of the intervention). Wardlaw JM, Murray V, Berge E, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet. 2012 Jun 23;379(9834):2364-72. Doi: 10.1016/S0140-6736(12)60738-7. Epub 2012 May 23. Review. PMID: 22632907; PMCID: PMC3386494. Wardlaw JM, Murray V, Berge E, del Zoppo GJ. Thrombolysis for Acute Ischemic Stroke, Update August 2014. Stroke. 2014;45(11):e222-e225. doi: 10.1161/STROKEAHA.114.007024. Berge E, Whiteley W, Audebert H, et al. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J. 2021 Mar;6(1):I-LXII. doi: 10.1177/2396987321989865. Epub 2021 Feb 19. PMID: 33817340; PMCID: PMC7995316. | ||
|
aPTT, activated partial thromboplastin time; ASPECTS, Alberta Stroke Program Early CT Score; CT, computed tomography; INR, international normalized ratio; NIHSS, National Institutes of Health Stroke Scale; PO, oral; rtPA, recombinant tissue plasminogen activator. | ||
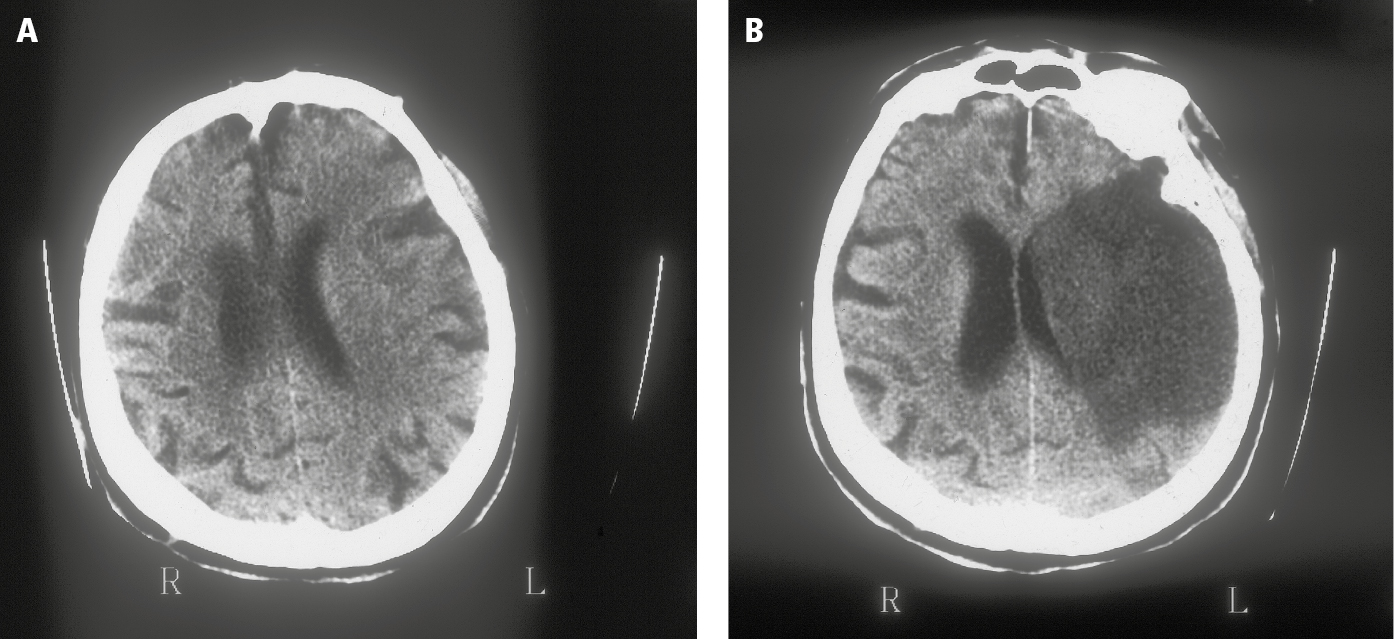
Figure 12.4-1. Computed tomography (CT) of ischemic stroke: A, left hemispheric edema with compression of the lateral ventricle, a hypodense area of the middle cerebral artery (MCA) territory, 3 hours following the onset of symptoms; B, a large ischemic lesion with midline shift, 72 hours following the onset.
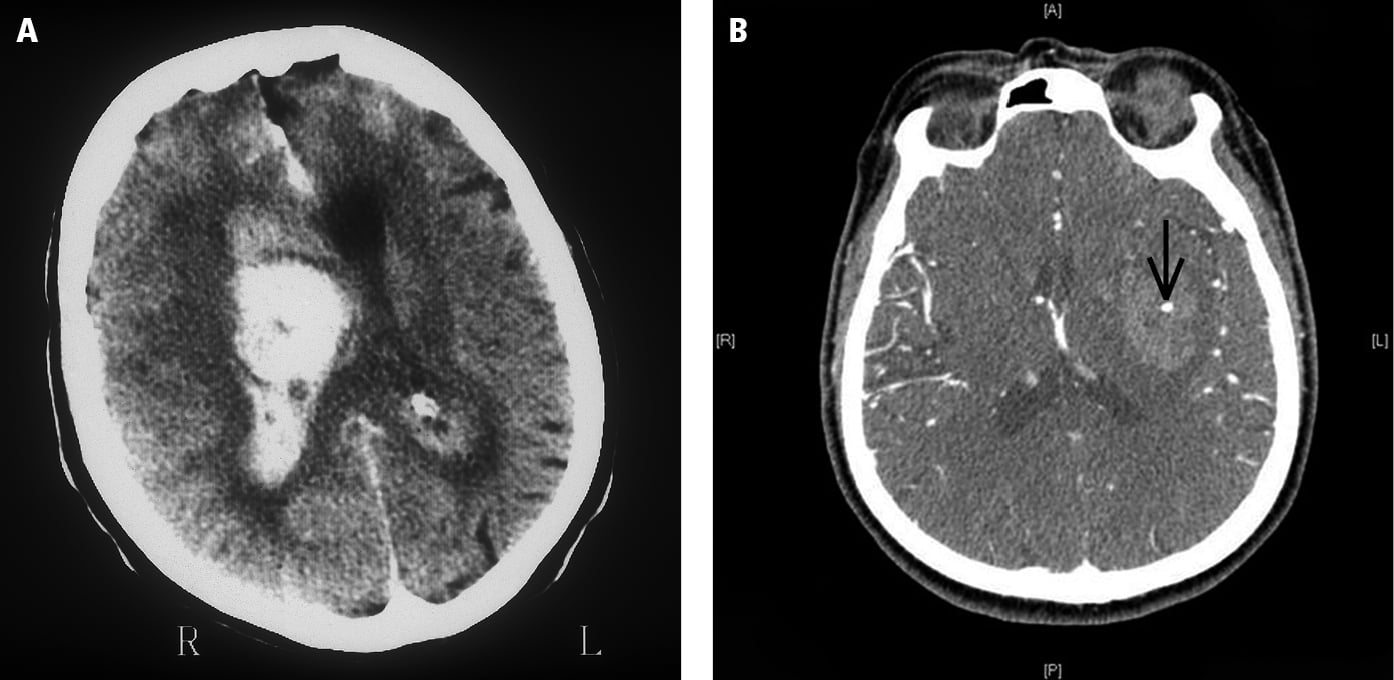
Figure 12.4-2. A, computed tomography (CT) of intracerebral hemorrhage to the right hemisphere with edema and midline shift; B, contrast-enhanced CT of the spot sign (arrow), indicating persisting bleeding.