Stevens SM, Woller SC, Kreuziger LB, et al. Antithrombotic Therapy for VTE Disease: Second Update of the CHEST Guideline and Expert Panel Report. Chest. 2021 Dec;160(6):e545-e608. doi: 10.1016/j.chest.2021.07.055. Epub 2021 Aug 2. Erratum in: Chest. 2022 Jul;162(1):269. doi: 10.1016/j.chest.2022.05.028. PMID: 34352278.
Martin KA, Beyer-Westendorf J, Davidson BL, Huisman MV, Sandset PM, Moll S. Use of direct oral anticoagulants in patients with obesity for treatment and prevention of venous thromboembolism: Updated communication from the ISTH SSC Subcommittee on Control of Anticoagulation. J Thromb Haemost. 2021 Aug;19(8):1874-1882. doi: 10.1111/jth.15358. Epub 2021 Jul 14. PMID: 34259389.
Ortel TL, Neumann I, Ageno W, et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 2020 Oct 13;4(19):4693-4738. doi: 10.1182/bloodadvances.2020001830. PMID: 33007077.
Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603. doi: 10.1093/eurheartj/ehz405. PMID: 31504429.
Konstantinides SV, Meyer G, Becattini C, et al; The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur Respir J. 2019 Oct 9;54(3):1901647. doi: 10.1183/13993003.01647-2019. PMID: 31473594.
Farge D, Frere C, Connors JM, et al; International Initiative on Thrombosis and Cancer (ITAC) advisory panel. 2019 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2019 Oct;20(10):e566-e581. doi: 10.1016/S1470-2045(19)30336-5. Epub 2019 Sep 3. PMID: 31492632.
Lim W, Le Gal G, Bates SM, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: diagnosis of venous thromboembolism. Blood Adv. 2018 Nov 27;2(22):3226-3256. doi: 10.1182/bloodadvances.2018024828. PMID: 30482764; PMCID: PMC6258916.
Kearon C, Akl EA, Ornelas J, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest. 2016 Feb;149(2):315-352. doi: 10.1016/j.chest.2015.11.026. Epub 2016 Jan 7. Erratum in: Chest. 2016 Oct;150(4):988. PMID: 26867832.
Zawilska K, Bała MM, Błędowski P, et al; Working Group from the Anticoagulation and Thrombolytic ACCP Conference. [Polish guidelines for the prevention and treatment of venous thromboembolism. 2012 update]. Pol Arch Med Wewn. 2012;122 Suppl 2:3-74. Polish. PMID: 23385605.
Definition, Etiology, PathogenesisTop
Deep vein thrombosis (DVT) refers to the development of a thrombus in the deep venous system (below the deep fascia) of the lower extremities or, less commonly, the pelvis or upper extremities. Thrombosis of other deep veins (eg, the portal vein) is considered a separate disease entity. Formation of a thrombus in the vein depends on the presence of ≥1 factor referred to as the Virchow triad, which includes:
1) Impaired blood flow (eg, due to immobilization of the limb or compression of the veins).
2) Procoagulant activity prevailing over the effects of coagulation inhibitors and fibrinolytic factors (congenital or acquired thrombophilia).
3) A damaged vascular wall (eg, as a result of injury during surgical procedures on a limb).
Risk factors:
1) Patient-related factors and clinical conditions: Age >40 (risk increases with age), obesity (body mass index >30 kg/m2), prior episodes of venous thromboembolism (VTE), trauma (particularly multiorgan trauma or fractures of the pelvis, proximal femur, and other long bones of the lower extremities), prolonged immobilization of the lower limb (eg, due to paresis, immobilization of two adjacent joints in a cast, general anesthesia [in particular involving muscle relaxants]), stroke that resulted in lower limb paresis, cancer (particularly pancreatic, brain, lung, ovarian, and kidney), family history of VTE, congenital or acquired thrombophilia (particularly deficiencies of antithrombin, protein C, and protein S, and antiphospholipid syndrome), sepsis, medical treatment of a severe debilitating disease (eg, severe pneumonia, including coronavirus disease 2019 [COVID-19] respiratory illness), New York Heart Association (NYHA) class III or IV heart failure (HF), respiratory failure, autoimmune diseases (eg, Crohn disease, ulcerative colitis, systemic lupus erythematosus), nephrotic syndrome, myeloproliferative neoplasms, paroxysmal nocturnal hemoglobinuria, venous compression (eg, due to tumor, hematoma, arterial malformation), pregnancy and postpartum period, a long flight (>6 h, particularly when sleeping in a sitting position), varicose veins of the lower extremities, severe infection.
2) Diagnostic, therapeutic, and prophylactic interventions: Major surgery, particularly involving the lower extremities, pelvis, and abdomen; an indwelling catheter in the large veins (particularly in the femoral vein); cancer treatment (chemotherapy, hormone therapy, and particularly the use of angiogenesis inhibitors); use of oral contraceptives, hormone replacement therapy, or selective estrogen receptor modulators; use of erythropoietin-stimulating agents.
Some risk factors are transient (surgery, trauma, temporary immobilization in a cast), while other are permanent (eg, congenital thrombophilia).
Causes of upper extremity DVT: An indwelling central venous catheter (the most frequent cause); compression of the subclavian or axillary vein by enlarged lymph nodes; malignant infiltrate; fractured clavicle; compression of the veins by the scalene muscles between the clavicle and the tendon of the subclavius muscle or the residual tendon band in the axillary fossa associated with strenuous physical activity or bodybuilding (Paget-Schrötter syndrome).
Clinical Features and Natural HistoryTop
1. Lower extremity DVT. Types:
1) Distal DVT involves ≥1 lower leg vein (peroneal, anterior tibial, posterior tibial). It is often asymptomatic, typically resolves spontaneously, and is associated with a low risk of clinically significant pulmonary embolism (PE), but it can progress to proximal vein thrombosis, typically in patients with ongoing VTE risk factors.
2) Proximal DVT involves ≥1 of the popliteal, superficial femoral, or common femoral veins, infrequently involves the iliac veins (~5%), and rarely extends into the inferior vena cava (<5%). It is usually symptomatic and carries a high risk of PE if untreated. Extensive DVT is a subgroup of proximal DVT that involves the iliofemoral veins and may warrant a different treatment approach than less extensive DVT. Massive DVT is a clinical distinction that refers to DVT manifesting with limb-threatening venous limb ischemia (phlegmasia cerulea dolens); most cases of massive DVT involve the iliofemoral veins. Submassive DVT is the most common form of DVT (>90% of cases) and is not limb threatening.
3) Phlegmasia dolens (Figure 3.20-1): An acute form of DVT involving the majority of the veins of the limb, accompanied by pain and massive edema.
a) Phlegmasia alba dolens: Severe edema, spasm of the arterioles in the skin, impaired capillary flow.
b) Phlegmasia cerulea dolens: The most severe form of DVT associated with an increased risk of amputation or death. Patients develop occlusion of nearly all veins in the limb, which leads to a significant increase in venous pressure and impairment of blood flow in the congested vascular bed, thus resulting in tissue hypoxia.
Signs and symptoms: DVT can be asymptomatic or may manifest with minor symptoms only. The patient may complain of calf pain while walking. Edema of the lower leg or the entire limb may be seen, sometimes perceived as tightness in the leg muscles, in which case it is necessary to compare the leg circumference of both legs ~5 cm below the tibial tuberosity (in unilateral thrombosis the difference is ≥2 cm); 70% of cases of unilateral edema of the lower limb are caused by DVT. Bilateral leg edema may be due to thrombosis of the inferior vena cava or conditions unrelated to thrombosis, such as HF, but it is less often related to bilateral leg DVT. Tenderness or pain on palpation may be present, with some patients complaining of limb pain at rest and in rare cases developing the Homans sign (calf pain that occurs with passive dorsal flexion of the foot). The limb may be warm and dilation of the superficial veins may persist with the limb elevated at a 45-degree angle. Low-grade or sometimes high-grade fever may be seen (due to inflammation of the tissues adjacent to the thrombotic vein). In patients with phlegmasia alba dolens the skin of the limb is pale. Phlegmasia cerulea dolens causes massive edema and severe pain at rest; the limb (usually the foot) initially turns cyanotic and subsequently, with the development of necrosis, its color changes to black.
2. Upper extremity DVT usually involves the axillary and subclavian veins. Limb edema and pain are the predominant symptoms.
3. Complications of DVT: Deep venous thrombi may undergo fragmentation, thus becoming embolic material that is carried into the pulmonary circulation.
1) A newly formed deep venous thrombus may detach from the vascular wall or undergo fragmentation and subsequently be carried to the lungs and cause PE. A massive PE may block pulmonary blood flow and cause cardiac arrest, which may be the initial manifestation of DVT. Undiagnosed and untreated DVT can be complicated by recurrent PE caused by small fragments of the thrombi that embolize and frequently is misdiagnosed as pneumonia or asthma.
2) Rarely, DVT may cause stroke or systemic embolism as a result of paradoxical embolism in patients with a functional right-to-left cardiac shunt (eg, a patent foramen ovale).
3) Long-term complications of DVT include postthrombotic syndrome (chronic venous insufficiency) as well as pulmonary hypertension as a complication of PE. In approximately two-thirds of patients treated for DVT the thrombus undergoes organization and the affected vein is partially recanalized (a total dissolution of the thrombus occurs only in a third of patients). This leads to chronic venous insufficiency and postthrombotic syndrome: organization of the thrombus results in damage to the venous valves, retrograde flow of venous blood, and eventually in venous hypertension.
DiagnosisTop
Due to the subtle and nonspecific clinical manifestations of VTE, it should be considered in patients with suggestive clinical features, especially when an alternative diagnosis is unlikely or has been excluded. Whenever in doubt, attempt to confirm or exclude the diagnosis of DVT because the disease is associated with a high risk of complications (including death) if left untreated. A prompt diagnosis of VTE allows the initiation of anticoagulant therapy, which is highly effective in preventing VTE-related sequelae.
1. Measurement of plasma D-dimer levels: The test performed to exclude DVT and PE (the reference range and cutoff values depend on the assay; most frequently thrombosis is unlikely in patients with D-dimer levels <500 microg/L). The diagnosis of VTE cannot be made on the basis of an increase in D-dimer levels alone, because an elevated D-dimer level is nonspecific and can occur in the elderly and in patients with other conditions not associated with thrombosis (eg, cancer, infection, inflammation). However, in patients with a low to intermediate clinical suspicion for DVT (assessed using a validated clinical prediction guide, such as the Wells score), a normal D-dimer value when using a high-sensitivity D-dimer assay (eg, enzyme-linked immunosorbent assay [ELISA]) is associated with a low likelihood of DVT (≤2%).
2. Compression ultrasonography (CUS) is the key method of confirming proximal vein thrombosis. A positive result means that a vein filled with thrombi does not collapse when compressed by the transducer. Ultrasonography of the entire deep vein system of the limb allows for the detection of distal thrombosis. In patients who have below-knee CUS for detection of distal thrombosis, this should be done by an experienced vascular laboratory, since below-knee CUS may produce a high proportion of false-positive and false-negative results.
In the case of suspected DVT always attempt to confirm or exclude the diagnosis to allow a prompt initiation of anticoagulant therapy (if confirmed) and avoidance of anticoagulation and associated bleeding risk (if excluded).
The diagnosis is based on a combination of the assessment of the clinical probability of thrombosis (eg, using the Wells score: Table 3.20-1), measurements of D-dimer levels, and/or CUS. If the diagnosis based on ultrasonography is doubtful, repeat the study; in exceptional cases, consider magnetic resonance venography or computed tomography (CT) venography.
1. Outpatients:
1) Low clinical probability of DVT: Measure D-dimer levels using a high-sensitivity (~95%) or moderate-sensitivity (~85%) assay. A negative D-dimer result is sufficient to exclude DVT.Evidence 1Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of intervention). Bates SM, Jaeschke R, Stevens SM, et al; American College of Chest Physicians. Diagnosis of DVT: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012 Feb;141(2 Suppl):e351S-418S. doi: 10.1378/chest.11-2299. PMID: 22315267; PMCID: PMC3278048. In patients with a positive result perform CUS. If CUS is negative, no further testing is needed.Evidence 2Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). Moderate Quality of Evidence (moderate confidence that we know true effects of intervention). Quality of Evidence lowered due to imprecision. Bates SM, Jaeschke R, Stevens SM, et al; American College of Chest Physicians. Diagnosis of DVT: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012 Feb;141(2 Suppl):e351S-418S. doi: 10.1378/chest.11-2299. PMID: 22315267; PMCID: PMC3278048.
2) Intermediate clinical probability of DVT: Measure D-dimer levels using a high-sensitivity (~95%) assay. A negative D-dimer result is sufficient to exclude thrombosis.Evidence 3Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of intervention). Bates SM, Jaeschke R, Stevens SM, et al; American College of Chest Physicians. Diagnosis of DVT: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012 Feb;141(2 Suppl):e351S-418S. doi: 10.1378/chest.11-2299. PMID: 22315267; PMCID: PMC3278048. In patients with a positive result perform CUS. If CUS is negative, repeat the study after 5 to 7 days.Evidence 4Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). Moderate Quality of Evidence (moderate confidence that we know true effects of intervention). Quality of Evidence lowered due to imprecision. Bates SM, Jaeschke R, Stevens SM, et al; American College of Chest Physicians. Diagnosis of DVT: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012 Feb;141(2 Suppl):e351S-418S. doi: 10.1378/chest.11-2299. PMID: 22315267; PMCID: PMC3278048.
3) High clinical probability of DVT or intermediate probability of DVT without the possibility to measure D-dimer levels using a high-sensitivity (~95%) assay: Perform CUS. If the result is negative, repeat the study after 5 to 7 days.Evidence 5Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). Moderate Quality of Evidence (moderate confidence that we know true effects of intervention). Quality of Evidence lowered due to imprecision. Bates SM, Jaeschke R, Stevens SM, et al; American College of Chest Physicians. Diagnosis of DVT: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012 Feb;141(2 Suppl):e351S-418S. doi: 10.1378/chest.11-2299. PMID: 22315267; PMCID: PMC3278048.
2. Hospitalized patients: It is necessary to perform CUS due to the low specificity and predictive value of positive D-dimer test results (the level is increased in many hospitalized patients, eg, due to major trauma, surgery, cancer, or inflammation), and sometimes also reduced sensitivity of the assay (due to the use of anticoagulants or the assay being performed a few days after the onset of clinical manifestations). In patients with a negative CUS result and a high probability of DVT, repeat CUS after 5 to 7 days. In patients with a lower probability of DVT, measure the D-dimer level and if it is positive, repeat CUS.
Limb injury (most frequent), chronic venous insufficiency (dysfunction of the venous valves, venous muscle pump, and plantar venous pump), superficial vein thrombosis, a Baker cyst (the posterior protrusion of the popliteal bursa [eg, due to trauma or in association with rheumatoid arthritis], which may rupture or compress the popliteal vein; this condition may cause thrombosis upon severe compression or as a result of local inflammation), cellulitis or lymphangitis, drug-induced edema (particularly in patients treated with calcium-channel blockers; edema is usually bilateral), lymphedema (in a third of patients with severe chronic venous insufficiency), hematomas of the lower leg muscles, plantaris muscle tendon rupture, myositis, tendinitis (particularly involving the Achilles tendon), or arthritis.
TreatmentTop
1. Treatment of symptomatic and asymptomatic DVT is the same. Management algorithm: Figure 3.20-2.
2. Conventional anticoagulant therapy is used for patients with clinically submassive DVT, whereas thrombolytic therapy is considered for patients with clinically massive DVT. Submassive DVT is characterized by proximal vein thrombosis, which may extend into the iliofemoral vein segment, but it does not produce severe symptoms or signs. Massive DVT, which typically involves the iliofemoral veins, is characterized by severe symptoms and signs that include extensive entire leg swelling, phlegmasia cerulea dolens, and limb ischemia.
3. A patient with acute submassive DVT does not have to be hospitalized and treatment can be started at home (using low-molecular-weight heparin [LMWH]) provided the following conditions are fulfilled:
1) The patient is clinically stable with normal vital signs.
2) There are no severe symptoms (severe pain or major edema of the lower limbs).
3) The risk of bleeding is low.
4) The patient has a creatinine clearance >30 mL/min.
5) Appropriate follow-up care is provided.
4. Early mobilization (this applies to the majority of patients): The patient should remain in bed with the limb elevated (with the lower leg in a horizontal position, the thigh directed at an angle towards the pelvis, and the limb supported along its whole length) only on the day the diagnosis of DVT is made and heparin treatment started.
5. Treatment with graduated compression: Elastic compression stockings (pantyhose, stockings, or knee-high socks are available; in most cases knee-high socks are sufficient) should not be routinely used in all patients with DVT but should be considered in patients with edema or other swelling. Typically elastic compression stockings are initiated during the first week after diagnosis when the associated pain and inflammation have subsided enough to allow the patient to comfortably wear the stockings. The patient should wear a knee-high sock or stocking (or bandage) throughout the day and walk as much as possible. At night it should be taken off, and the bed mattress in the region of the leg should be elevated by 10 to 15 cm. Contraindications to compression treatment include phlegmasia cerulea dolens, concomitant limb ischemia due to arterial disease (measure the ankle-brachial index [ABI] or at least make sure that the pulse assessed on the dorsalis pedis artery and posterior tibial artery is present and symmetric on both lower extremities), cellulitis or frail skin with loss of subcutaneous tissue related to advanced age, or systemic glucocorticoid use.
6. Anticoagulant treatment (see below); this treatment modality is of key importance.
7. Placement of inferior vena cava filters should be considered in patients with acute proximal DVT of the lower extremities if the use of anticoagulants at therapeutic doses is contraindicated (because of the risk of bleeding or need for a major surgical procedure that cannot be postponed) or ineffective (recurrent PE or significant enlargement of the thrombus despite adequate anticoagulant treatment). Retrievable filters, which are typically removed after 1 to 3 weeks, are preferred. Anticoagulant treatment should be started or resumed once the risk of bleeding has decreased.
8. Thrombolysis: Consider systemic thrombolysis for patients with clinically massive DVT that may be accompanied by phlegmasia cerulea dolens, and only if local infusion of a thrombolytic agent via a catheter is not feasible. Local thrombolysis may be beneficial in the following groups of patients:
1) Patients with clinically massive DVT (eg, iliofemoral DVT with severe edema and pain), <14 days from the onset of symptoms, who are in good general condition (low risk of bleeding).
2) Patients with early upper extremity DVT (<14 days from the symptom onset) or at risk of amputation.
Thrombolytic agents are administered locally via a catheter inserted into the thrombus. This is preferably combined with mechanical fragmentation of the thrombus and aspiration of its fragments. Following successful thrombolysis, use the same anticoagulant treatment as in similar patients receiving medical treatment only.
Initial Anticoagulant Treatment
1. General principles of anticoagulant treatment in VTE: Figure 3.20-3.
2. In patients with a high or intermediate clinical probability of DVT or with confirmed DVT, start anticoagulant treatment immediately once contraindications have been excluded, even if the results of diagnostic tests are not yet available. If the tests cannot be performed promptly and the probability of DVT is at least intermediate, start treatment before the diagnosis is established.
In nonhospitalized patients with acute isolated distal lower extremity DVT (veins of the lower leg [peroneal vein; anterior and posterior tibial veins]), it is suggested to start anticoagulant therapy if there are severe symptoms or risk factors for thrombus extension (eg, positive D-dimer test results, active cancer, immobility, thrombus in close proximity to the proximal veins, thrombus >5 cm in length).
In patients who have mild to moderate symptoms and do not have risk factors for thrombus extension, it is suggested to withhold anticoagulant therapy and undertake serial CUS over 2 weeks. If there is worsening of symptoms or evidence of thrombus extension into the proximal veins on ultrasonography testing, it is suggested to start anticoagulant therapy.Evidence 6Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). Low Quality of Evidence (low confidence that we know true effects of the intervention). Quality of Evidence lowered due to the risk of bias and imprecision. Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012 Feb;141(2 Suppl):e419S-94S. doi: 10.1378/chest.11-2301. Erratum in: Chest. 2012 Dec;142(6):1698-1704. PMID: 22315268; PMCID: PMC3278049. Righini M, Galanaud JP, Guenneguez H, et al. Anticoagulant therapy for symptomatic calf deep vein thrombosis (CACTUS): a randomised, double-blind, placebo-controlled trial. Lancet Haematol. 2016 Dec;3(12):e556-e562. doi: 10.1016/S2352-3026(16)30131-4. Epub 2016 Nov 8. PMID: 27836513.
3. LMWH, unfractionated heparin (UFH), fondaparinux, rivaroxaban, apixaban, edoxaban, or dabigatran: LMWH or fondaparinux are preferred over UFH due to the convenient dosage and no need for laboratory monitoring of the anticoagulant effects. In patients treated with a vitamin K antagonist (VKA) who have developed acute lower extremity DVT, start parenteral anticoagulant treatment (LMWH, fondaparinux, or UFH). In patients with kidney failure (creatinine clearance <30 mL/min), UFH is preferred (you may also reduce the dose of LMWH by 50% or monitor plasma anti-Xa levels). In certain clinical situations (eg, patients at risk of hemorrhagic complications, patients for whom thrombolytic therapy is considered or urgent surgery is likely) starting therapy with UFH is preferred because of its shorter duration of action and the possibility of easy neutralization of anticoagulant effects with protamine when necessary. In patients at risk of heparin-induced thrombocytopenia (HIT) use fondaparinux (see Heparins). Dosage:
1) Subcutaneous LMWH at a therapeutic dose every 12 hours (initial treatment) or every 24 hours (in long-term and outpatient treatment) (Table 3.20-2). If the clinical efficacy of LMWH is questionable (eg, thrombus progression is seen), measure the anti-Xa level (preferably 4 h after the last LMWH administration; target anti-Xa levels are 0.6-1.0 IU/mL in patients receiving LMWH every 12 h and 1-1.3 IU/mL in those receiving LMWH once daily). If anti-Xa measurement is not feasible, administer IV UFH and monitor the activated partial thromboplastin time (aPTT).
2) UFH:
a) IV administration: Inject 80 IU/kg (or up to 5000 IU) as an IV bolus followed by a continuous IV infusion at the rate of 18 IU/kg/h (or up to 1300 IU/h). Assess aPTT after 6 hours. If the aPTT value is within the therapeutic range (1.5-fold to 2.5-fold prolongation compared with the reference value; usually aPTT in the course of treatment should be 60-90 seconds), continue the infusion at the same dose (the average maintenance dose is 25,000-35,000 IU/d); otherwise increase or decrease the dose of UFH accordingly (Table 3.20-3).
b) Subcutaneous administration: If the anticoagulant effect is being monitored, use a concentrated formulation of 25,000 IU/mL, initially 80 IU/kg IV followed by 250 IU/kg subcutaneously every 12 hours, and adjust the doses so that 6 hours after the injection of the drug aPTT remains in the therapeutic range (the average maintenance dose is 17,500 IU every 12 h). An alternative treatment option is to start with 333 IU/kg subcutaneously, followed after 12 h by 250 IU/kg subcutaneously every 12 h, which can be safely done without laboratory monitoring.Evidence 7Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due to imprecision. Kearon C, Ginsberg JS, Julian JA, et al; Fixed-Dose Heparin (FIDO) Investigators. Comparison of fixed-dose weight-adjusted unfractionated heparin and low-molecular-weight heparin for acute treatment of venous thromboembolism. JAMA. 2006 Aug 23;296(8):935-42. doi: 10.1001/jama.296.8.935. PMID: 16926353.
In patients who fail to achieve the target aPTT values despite the use of high-dose UFH, adjust the dose on the basis of the anti-Xa levels, with a targeted therapeutic range of 0.35 to 0.70 anti–factor Xa IU/mL.
3) Fondaparinux: Administer 7.5 mg subcutaneously every 24 hours. In patients with a body weight >100 kg, you may increase the dose to 10 mg every 24 hours.
4) Rivaroxaban: Administer 15 mg orally bid for 3 weeks, followed by 20 mg once daily (15 mg if GFR <50 mL/min; do not use if GFR <30 mL/min).
5) Apixaban: Administer 10 mg orally bid for the first 7 days, then 5 mg bid. In long-term treatment (>3 months) use 2.5 mg bid.
6) Dabigatran: Switch from LMWH or UFH to dabigatran after 5 to 7 days of LMWH or UFH treatment. Administer dabigatran 150 mg orally bid (110 mg bid is suggested in patients at high risk of bleeding).
7) Edoxaban: Switch from LMWH or UFH to edoxaban after 5 to 7 days of LMWH or UFH treatment. Administer edoxaban 60 mg orally once daily (30 mg once daily is suggested in patients with moderate renal insufficiency [creatinine clearance 30-50 mL/min], body weight <60 kg, concomitant use of potent p-glycoprotein inhibitors).
4. Duration of heparin and fondaparinux treatment:
1) In the majority of patients for whom VKA use is planned, these agents should be administered from the first day of treatment. Discontinue heparin and fondaparinux if the international normalized ratio (INR) is ≥2.0 for 2 consecutive days of combination treatment with VKAs, but not earlier than after 5 days of the administration of heparin or fondaparinux. Do not discontinue heparin/fondaparinux on the day of starting the VKA, because in the first days the anticoagulant effect of VKAs is incomplete, and therefore the concomitant administration of heparin/fondaparinux is necessary.
2) In patients with extensive iliofemoral DVT causing severe edema and limb pain, use heparin or LMWH for 7 to 10 days (or longer, until symptom relief) and consider deferring the start of VKA administration.
3) In patients for whom VKAs are contraindicated or not recommended, continue LMWH. This pertains in particular to the following situations:
a) Pregnant women with VTE, since VKAs cross the placenta and may be harmful to the developing fetus.
b) Patients with cancer, as LMWHs are more effective and safer than VKAs. The agents should be used at least during the first 3 to 6 months of treatment. An alternative to VKAs for patients with cancer and VTE are the direct oral anticoagulants (DOACs) apixaban, edoxaban, and rivaroxaban, which are administered in the same manner as in patients without cancer.
c) When regular and accurate INR monitoring cannot be performed.
d) If the current episode of VTE occurred despite the administration of appropriate VKA doses.
5. Treatment with VKAs:
1) Start the administration of acenocoumarol or warfarin simultaneously with heparin or fondaparinux, usually on the first day of treatment. If you plan to use heparin for >7 days (see above), you may delay VKA administration.
2) In the first 2 days, use 6 mg of acenocoumarol or 10 mg of warfarin; do not use >6 mg of acenocoumarol or >10 mg of warfarin. In the elderly, debilitated, or malnourished patients, as well as in patients with HF, liver disease, those receiving drugs that enhance the effects of VKAs, or those at increased risk of bleeding, start treatment with 4 mg of acenocoumarol or 5 mg of warfarin.
3) Assess the INR on day 3 and adjust the dose on the basis of the results.
4) In patients with an INR ≥2.0 for 2 consecutive days, discontinue heparin/fondaparinux and continue treatment with a VKA alone for a time dependent on the risk of relapse (≥3 months; Table 3.20-4) at doses adjusted to maintain the INR within the range of 2.0 to 3.0.
5) Principles of safe VKA treatment (contraindications, monitoring, dose adjustment, management of complications): see Vitamin K Antagonists.
6. Treatment with rivaroxaban or apixaban:
1) Rivaroxaban or apixaban may be used from the beginning of DVT treatment.
2) Unlike with VKAs, it is not necessary to start treatment with the concomitant administration of heparin.
3) As the cost of apixaban and rivaroxaban treatment is higher than that of VKA, consider whether the patient will be able to continue therapy for several months. Note that due to its shorter duration of action, skipping 1 or 2 doses of a DOAC may have more serious consequences than in the case of VKAs.
7. Treatment with dabigatran:
1) Dabigatran is used only after 5 to 7 days of LMWH treatment.
2) Bearing in mind that the cost of dabigatran treatment is higher than that of VKA, consider whether the patient will be able to continue therapy for several months. Note that due to its shorter duration of action, skipping a dose of dabigatran may have more serious consequences than in the case of VKAs.
8. Treatment with edoxaban:
1) Edoxaban is used only after 5 to 7 days of LMWH treatment.
2) Bearing in mind that the cost of edoxaban treatment is higher than that of VKA treatment, consider whether the patient will be able to continue therapy for several months. Note that due to its shorter duration of action, skipping a dose of edoxaban may have more serious consequences than in the case of VKAs.
1. Patients with VTE require long-term anticoagulant treatment if there is a high risk of recurrent thrombosis and their risk for bleeding is not high. The risk is higher in the case of cancer, severe thrombophilia (eg, antithrombin deficiency, antiphospholipid antibody syndrome), elevated plasma D-dimer levels after anticoagulation, male sex, or history of recurrent VTE.
2. Methods of preventing VTE recurrence after upper or lower extremity DVT and after PE are similar. In the majority of patients best results are obtained with long-term administration of VKAs at a dose that maintains the INR in the range of 2.0 to 3.0. LMWHs are recommended in patients with cancer. The recommended duration of anticoagulant treatment depends on the clinical condition (Table 3.20-4) and the risk of bleeding (Table 3.20-5).
3. In patients with recurrent VTE despite maintaining the INR in the range of 2.0 to 3.0, consider the use of VKAs at a dose that maintains INR in the range of 2.5 to 3.5. This therapeutic range may be also more adequate for patients with antiphospholipid antibodies (APLAs) and additional VTE risk factors or with a thromboembolic event despite maintaining the INR in the range of 2.0 to 3.0, as well as in patients with an elevated baseline INR due to the presence APLAs.
4. If VKAs cannot be used (eg, if regular monitoring of anticoagulant effects is contraindicated or not feasible), administer a DOAC or LMWH subcutaneously.
5. To prevent recurrent VTE in the course of long-term treatment, you may consider switching from a VKA to apixaban 5 mg bid, rivaroxaban 20 mg once daily, or dabigatran 150 mg bid (consider using lower doses in patients at high risk of bleeding, comprising apixaban 2.5 mg bid, rivaroxaban 10 mg daily, or dabigatran 110 mg bid). Edoxaban has not been evaluated in randomized trials for long-term treatment but is a likely acceptable option at a dose of 60 mg daily.
6. Periodically evaluate the benefits and risks of anticoagulant treatment, as it reduces the risk of VTE recurrence but at the same time increases the risk of bleeding.
7. Compression therapy: Use class II compression elastic stockings (see Table 3.19-8) in patients with persistent edema or other leg swelling. In most cases knee-length socks are the best option when fitted to the size of the limb in accordance with the manufacturer’s recommendations.
1. Drugs (options, dosage: Table 3.20-6):
1) Subcutaneous LMWHs (preferred treatment) at doses adjusted to the body weight before pregnancy, administered until the end of pregnancy. Monitoring of anti-Xa levels every 1 to 3 months in the course of treatment is recommended (if available). Measure the anti-Xa level ~4 hours after the last injection of LMWH; the target levels are 0.6 to 1.0 IU/mL in patients receiving LMWHs every 12 hours and 1 to 1.3 IU/mL in those receiving LMWHs every 24 hours.
2) UFH (if LMWHs are not available):
a) IV administration (as initial treatment and in certain situations: see below): Inject 80 IU/kg (or up to 5000 IU) as an IV bolus followed by a continuous IV infusion at a dose that maintains the aPTT in the therapeutic range for 5 days (1.5-fold to 2.5-fold prolongation compared with the reference value; usually in the course of treatment aPTT should be 60-90 seconds), then treat the patient with subcutaneous LMWHs or UFH until the end of pregnancy.
b) Subcutaneous administration at an individually adjusted dose until the end of pregnancy: Start with the subcutaneous administration of 333 IU/kg followed after 12 hours by 250 IU/kg every 12 hours, and adjust the dose to maintain the aPTT within the therapeutic range 6 hours after the injection (the average maintenance dose is 17,500 IU every 12 h).
2. Use heparin at an individually adjusted dose for ≥3 months. Subsequently, you can reduce the dose by 25% to 50% without loss of efficacy, especially in women at increased risk of bleeding or osteoporosis.
3. Avoid DOACs in pregnancy and in women who are breastfeeding, as safety of these drugs has not been evaluated in these clinical settings.
4. Management at delivery: The delivery should be planned. In patients with VTE there is no preference for either cesarean section or induced vaginal delivery.
1) Before the planned induction of vaginal delivery or cesarean section, discontinue subcutaneous LMWH/UFH 24 hours prior to the scheduled time of delivery.
2) Before the planned induction of vaginal delivery or cesarean section in patients at a very high risk of VTE recurrence (eg, proximal lower extremity DVT within prior 4 weeks), switch from subcutaneous LMWH/UFH to a full therapeutic dose of IV UFH and then discontinue heparin 4 to 6 hours before the scheduled time of delivery. You may consider placing a retrievable inferior vena cava filter before the delivery and removing it afterwards (this may be used in special situations only, eg, VTE within 4 weeks, as sometimes the filter cannot be retrieved).
3) Spontaneous labor: In women receiving subcutaneous UFH closely monitor the aPTT. If it is significantly prolonged at labor, consider the administration of protamine (dosage: see Heparins).
4) Spinal or epidural anesthesia can be administered as long as the last dose of LMWH was 24 hours ago, the last dose of subcutaneous UFH was 12 hours ago, or IV UFH was stopped 4 to 6 hours ago.
5. Postdelivery management: For 6 weeks (or longer, so that the entire duration of anticoagulant treatment is ≥6 months) use VKAs at a dose that maintains the INR in the range of 2.0 to 3.0, initially in combination with LMWHs or UFH until INR ≥2.0 is achieved on 2 consecutive days.
Treatment of DVT in Patients with Obesity
With the worldwide rise in the prevalence of obesity, there is a corresponding increase in the need to manage patients with obesity who develop DVT. Treatment: see Pulmonary Embolism (PE).
PreventionTop
1. See Primary Prevention of Venous Thromboembolism.
2. Prevention of recurrent VTE by appropriate treatment of a VTE episode: see Duration of Treatment, above.
Tables and FiguresTop
|
Clinical feature |
Score |
|
Cancer (treated or diagnosed within the prior 6 months) |
1 |
|
Paralysis, paresis, or recent lower limb immobilization in a cast |
1 |
|
Recently bedridden for >3 days or major surgery within the prior 4 weeks |
1 |
|
Localized tenderness along distribution of the deep veins of the lower extremitya |
1 |
|
Edema of the entire lega |
1 |
|
Calf swelling >3 cm compared to the asymptomatic leg (measured 10 cm below the tibial tuberosity)a |
1 |
|
Pitting edema (more prominent on the symptomatic leg)a |
1 |
|
Visible collateral superficial veins (nonvaricose)a |
1 |
|
Other diagnosis as likely or more likely than deep vein thrombosis |
–2 |
|
Interpretation Low clinical probability: Total score ≤0 Intermediate clinical probability: Total score 1-2 High clinical probability: Total score ≥3 |
|
|
a If symptoms occur in both lower limbs, the limb in which symptoms are more severe should be assessed. |
|
|
Adapted from Lancet. 1997;350(9094):1795-8 and N Engl J Med. 2003;349(13):1227-35. |
|
|
Low-molecular-weight heparin |
Dosage |
|
|
Twice daily |
Once daily |
|
|
Dalteparin |
100 IU/kg every 12 h |
200 IU/kg every 24 h |
|
Enoxaparin |
1 mg/kg every 12 h |
1.5 mg/kg every 24 h |
|
Nadroparin |
85 IU/kg every 12 h |
170 IU/kg every 24 h |
|
Tinzaparin |
Not used |
175 IU/kg every 24 h |
|
aPTT (s)a |
IV bolus |
Continuous IV infusion |
|
First dose |
80 IU/kg |
18 IU/kg/h |
|
<35 (<1.2 × control) |
80 IU/kg |
Increase by 4 IU/kg/h |
|
35-45 (1.2-1.5 × control) |
40 IU/kg |
Increase by 2 IU/kg/h |
|
46-70 (1.5-2.5 × control)b |
Without IV injection |
No change |
|
71-90 (2.5-3 × control) |
Without IV injection |
Reduce by 2 IU/kg/h |
|
>90 (>3 × control) |
Without IV injection |
Stop infusion for 1 h, then reduce by 3 IU/kg/h |
|
a Sample numerical values expressed in seconds may vary depending on the reference (control) values in a given laboratory. b The therapeutic aPTT range of 46-70 s should correspond to the anti-Xa activity of 0.3-0.7 IU/mL. Repeat aPTT and adjust the unfractionated heparin dose after 6 hours. |
||
|
aPTT, activated partial thromboplastin time; IV, intravenous. |
||
|
3 months |
|
– Proximal lower extremity DVT or pulmonary embolism caused by surgery or other transient risk factor – Upper extremity DVT associated with a central venous catheter that has already been removed in patients with or without cancer – Upper extremity DVT unrelated to a central venous catheter or to cancer – First episode of VTE in the form of idiopathic proximal lower extremity DVT or idiopathic pulmonary embolism in patients at high risk of bleedinga – First episode of isolated distal lower-extremity DVT – Second episode of idiopathic VTE in patients at high risk of bleedinga |
|
>3 monthsb |
|
– Upper extremity DVT associated with a central venous catheter that has not yet been removed (anticoagulant treatment should be administered as long as the catheter remains in the central vein or for 3 months if the catheter is removed) – Lower extremity DVT and active cancer (metastatic or treated within the prior 6 months) – First episode of VTE in the form of idiopathic proximal lower extremity DVT in patients at low or moderate risk of bleedinga – Second episode of idiopathic VTE in patients at low or moderate risk of bleedinga |
|
a Risk factors for bleeding: see table 3.20-5. b The need for continued treatment should be assessed periodically (eg, once a year). |
|
DVT, deep vein thrombosis; VTE, venous thromboembolism. |
|
Factors associated with bleeding during anticoagulant therapy: – Older age (>65 years and particularly >75 years)a – Previous bleeding (particularly if the cause was not correctable) – Cancer (particularly if metastatic or highly vascular) – Renal insufficiency – Liver failure – Diabetes mellitus – Previous stroke – Thrombocytopenia – Anemia – Concomitant antiplatelet therapy – Recent surgery – Frequent falls – Alcohol abuse – Reduced functional capacity – Poor control of VKA therapy |
|
With an increase in the severity of individual factors and with an increase in the number of factors present, the risk of bleeding is expected to increase (both at baseline and while on anticoagulants). |
|
a Young (eg, <65 years) healthy patients with good VKA control will have a low risk of major bleeding (≤1% per patient-year), those with less severe factors have an intermediate risk, and elderly patients with severe or multiple factors are at high risk for major bleeding (>4% per patient-year). |
|
Based on Blood. 2014;123(12):1794-801. |
|
VKA, vitamin K antagonist. |
|
Adjusted dose |
|
|
Unfractionated heparin |
Dose to maintain aPTT within therapeutic range, SC administration, 333 IU/kg initially, followed by 250 IU/kg every 12 h |
|
Dalteparin |
100 IU/kg SC every 12 h or 200 IU/kg SC every 24 h |
|
Enoxaparin |
1 mg/kg SC every 12 h or 1.5 mg/kg SC every 24 h |
|
Nadroparin |
85 IU/kg SC every 12 h or 190 IU/kg SC every 24 h |
|
Tinzaparin |
175 IU/kg SC every 24 h |
|
aPTT, activated partial thromboplastin time; SC, subcutaneous. |
|
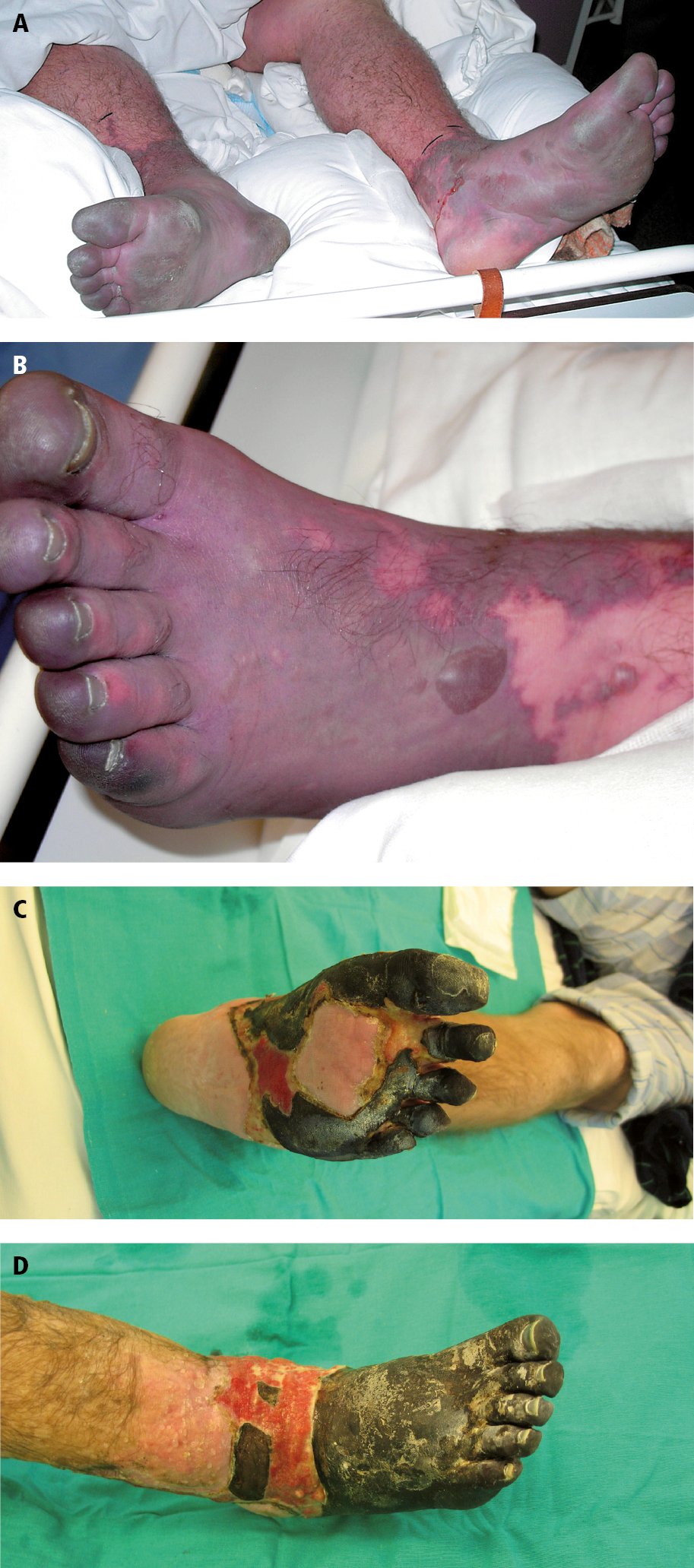
Figure 3.20-1. Phlegmasia cerulea dolens: A, a late phase, following edema resolution; B-D, development of necrosis. Figure courtesy of Dr Rafał Niżankowski (A-B) and Dr Marzena Frołow (C-D).
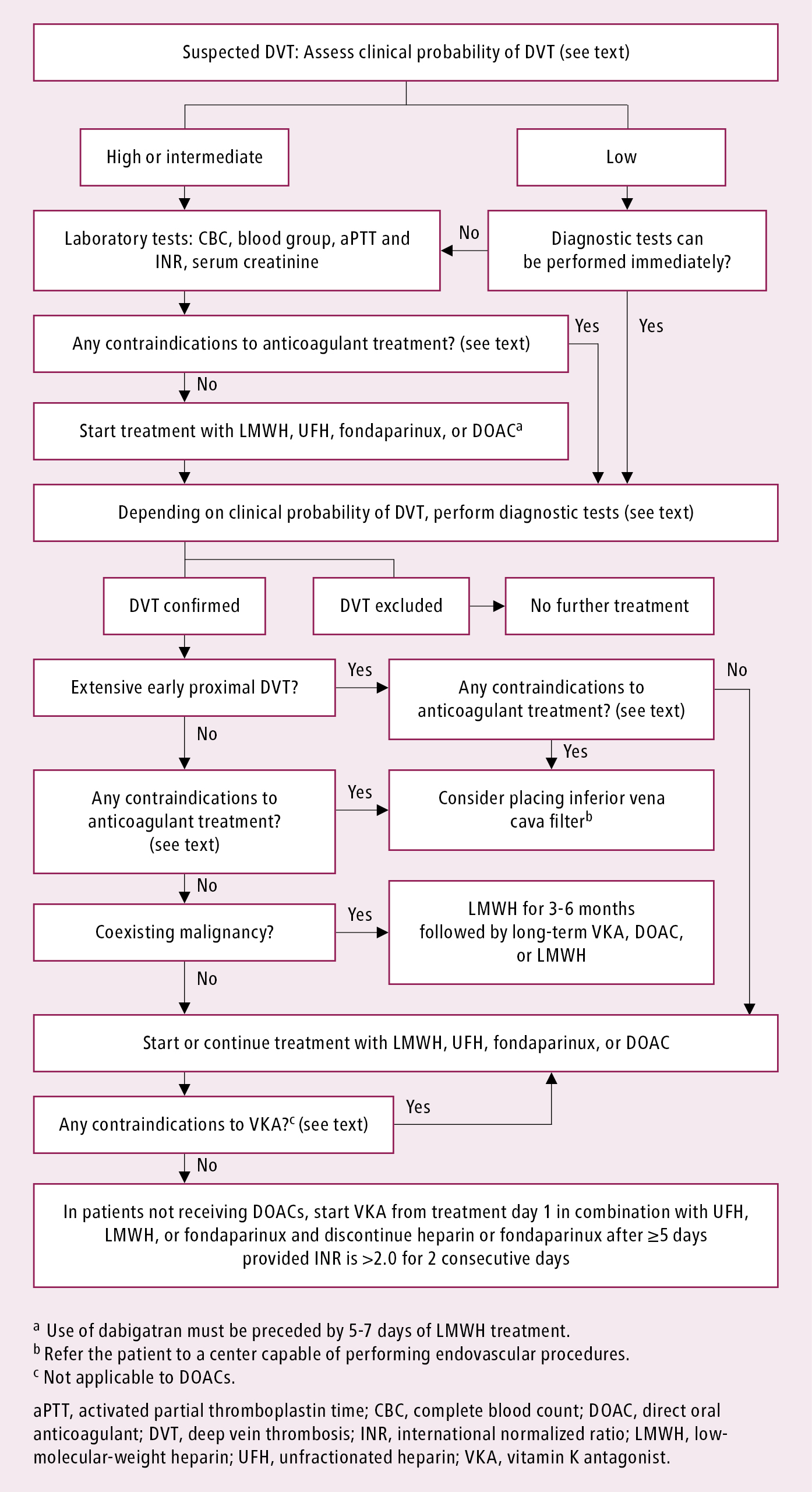
Figure 3.20-2. Management algorithm of lower extremity deep venous thrombosis.
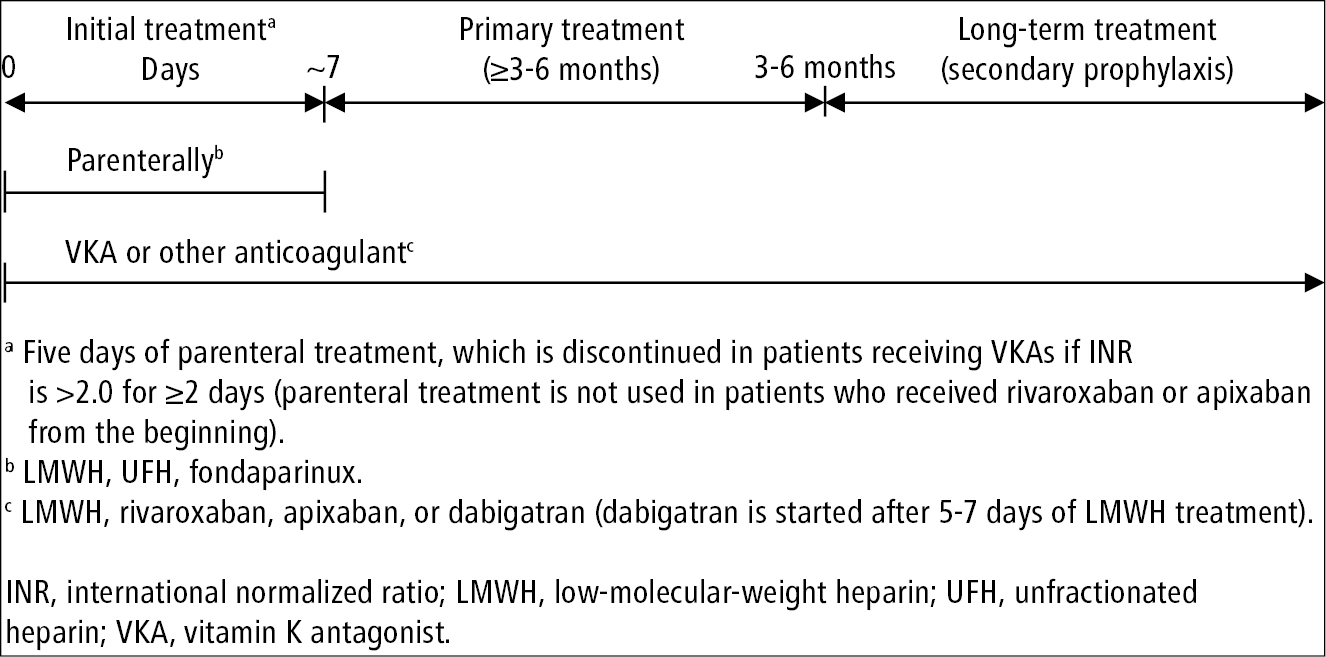
Figure 3.20-3. General principles of anticoagulant treatment in patients with venous thromboembolism. Based on the 2020 American Society of Hematology guidelines.