American Diabetes Association. Standards of Care in Diabetes–2023. Diabetes Care. 2023;46(Suppl 1):S1-S280.
Cook O, Prebtani APH. Quick Guide to SGLT-2 Inhibitors (SGLT-2i) for Glycemic Control or Renal Protection or Cardiovascular Protection. Accessed March 8, 2023. https://empendium.com/mcmtextbook/bonus-resources/307813,sglt-2-inhibitors-a-quick-guide
Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2022 Dec;65(12):1925-1966. doi: 10.1007/s00125-022-05787-2. Epub 2022 Sep 24. PMID: 36151309; PMCID: PMC9510507.
Li S, Vandvik PO, Lytvyn L, et al. SGLT-2 inhibitors or GLP-1 receptor agonists for adults with type 2 diabetes: a clinical practice guideline. BMJ. 2021 May 11;373:n1091. doi: 10.1136/bmj.n1091. PMID: 33975892.
Palmer SC, Tendal B, Mustafa RA, et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ. 2021 Jan 13;372:m4573. doi: 10.1136/bmj.m4573. PMID: 33441402; PMCID: PMC7804890.
Diabetes Canada Clinical Practice Guidelines Expert Committee; Lipscombe L, Butalia S, Dasgupta K, et al. Pharmacologic Glycemic Management of Type 2 Diabetes in Adults: 2020 Update. Can J Diabetes. 2020 Oct;44(7):575-591. doi: 10.1016/j.jcjd.2020.08.001. PMID: 32972640.
Diabetes Canada Clinical Practice Guidelines Steering Committee; Senior PA, Houlden RL, Kim J, et al. Pharmacologic Glycemic Management of Type 2 Diabetes in Adults: 2020 Update - The User's Guide. Can J Diabetes. 2020 Oct;44(7):592-596. doi: 10.1016/j.jcjd.2020.08.002. PMID: 32972641.
Araszkiewicz A, Bandurska-Stankiewicz E, Budzyński A, et al. 2019 Guidelines on the management of diabetic patients. A position of Diabetes Poland. Clinical Diabetology. 2019;8(1):1-95. doi: 10.5603/DK.2019.0001.
Diabetes Canada Clinical Practice Guidelines Expert Committee. Diabetes Canada 2018 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Can J Diabetes. 2018;42(Suppl 1):S1-S325.
Davies MJ, D'Alessio DA, Fradkin J, et al. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018 Dec;41(12):2669-2701. doi: 10.2337/dci18-0033. Epub 2018 Oct 4. PubMed PMID: 30291106; PubMed Central PMCID: PMC6245208.
Diabetes Canada Clinical Practice Guidelines Expert Committee; Prebtani APH, Bajaj HS, Goldenberg R, Mullan Y. Reducing the Risk of Developing Diabetes. Can J Diabetes. 2018 Apr;42 Suppl 1:S20-S26. doi: 10.1016/j.jcjd.2017.10.033. PMID: 29650097.
Siu AL; U.S. Preventive Services Task Force. Screening for Abnormal Blood Glucose and Type 2 Diabetes Mellitus: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015 Dec 1;163(11):861-8. doi: 10.7326/M15-2345. Epub 2015 Oct 27. PubMed PMID: 26501513
Evert AB, Boucher JL, Cypress M, et al; American Diabetes Association. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2013 Nov;36(11):3821-42. doi: 10.2337/dc13-2042. Epub 2013 Oct 9. PubMed PMID: 24107659; PubMed Central PMCID: PMC3816916.
Canadian Task Force on Preventive Health Care, Pottie K, Jaramillo A, Lewin G, et al. Recommendations on screening for type 2 diabetes in adults. CMAJ. 2012 Oct 16;184(15):1687-96. doi: 10.1503/cmaj.120732. Erratum in: CMAJ. 2012 Nov 6;184(16):1815. PubMed PMID: 23073674; PubMed Central PMCID: PMC3478353.
Lipsky BA, Berendt AR, Cornia PB, et al; Infectious Diseases Society of America. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012 Jun;54(12):e132-73. doi: 10.1093/cid/cis346. PubMed PMID: 22619242.
Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012 Jun;35(6):1364-79. doi: 10.2337/dc12-0413. Epub 2012 Apr 19. Review. Erratum in: Diabetes Care. 2013 Feb;36(2):490. PubMed PMID: 22517736; PubMed Central PMCID: PMC3357214.
Definition, Etiology, PathogenesisTop
Diabetes mellitus (DM) is a group of metabolic disorders in which various genetic and environmental factors result in the progressive loss of beta-cell mass or function (or both) that manifests clinically as hyperglycemia. Chronic hyperglycemia in the course of DM is associated with damage, dysfunction, and failure of multiple organs—particularly the eyes, kidneys, peripheral and autonomic nerves, heart, small and large blood vessels—and increases the risk of cardiovascular and cerebrovascular diseases as well as cognitive decline.
1. Type 1 DM is caused by the destruction of pancreatic beta cells due to an autoimmune process (type 1A, associated with beta cell autoantibodies) or due to unknown mechanisms (idiopathic or type 1B) that typically results in absolute insulin deficiency. Type 1A DM (5%-10% of all patients with diabetes) develops more frequently in children, adolescents, and younger adults, but it can occur at any age. The disease occurs in genetically susceptible individuals with particular gene polymorphisms (human leukocyte antigen [HLA] associations, with linkage to DQA and DQB genes) and in many cases appears to be triggered by environmental factors (eg, perinatal events, viral infections, ingestion of cow’s milk). The autoantibodies (islet cell autoantibodies and autoantibodies to GAD-65 [the most sensitive and specific], insulin, tyrosine phosphatases IA-2 and IA-2beta, and ZnT8) may appear several years before symptoms of DM are observed. Their persistence is an almost certain predictor of DM. Age when antibodies are first detected, number of antibodies, antibody specificity, and antibody titers are the main factors that predict the rate of progression to DM. After disease onset and initiation of insulin therapy the process of beta cell destruction continues slowly, and patients usually have decreased insulin requirements, as beta cells are able to produce some insulin once the glucotoxic phase is over. This phase is termed the honeymoon phase and can last for months to years (in rare circumstances) until there is a complete destruction of beta cells.
There are 3 staging phases of type 1 DM that have been described:
1) Stage 1 is characterized by the presence of autoimmunity but with normal glucose levels and absence of symptoms.
2) Stage 2 is associated with glucose levels in the range of impaired fasting glucose (IFG; 6.1-6.9 mmol/L [100-125 mg/dL]) and/or impaired glucose tolerance (IGT; 2-hour plasma glucose of 7.8-11 mmol/L [140-199 mg/dL]) with glycated hemoglobin (HbA1c) between 6.0% and 6.4% (or ≥10% increase in HbA1c).
3) Stage 3 is characterized by the onset of symptoms with glucose levels meeting the criteria for the diagnosis of DM.
In some cases autoimmune destruction of beta cells leads to the onset of DM in older adults (latent autoimmune diabetes in adults [LADA]; Table 6.2-1). These patients initially appear to have type 2 DM but have positive circulating beta cell autoantibodies and progress to insulin dependence after a few months or years. LADA includes a heterogeneous group of patients, with some having high titers of beta cell autoantibodies and progressing to insulin dependence faster. The disappearance of serum C-peptide (see Diagnostic Tests, below) indicates a total destruction of beta cells.
2. Type 2 DM is the most common form of DM (~90% of patients). It is characterized by varying degrees of insulin resistance coexisting with progressive impairment of insulin secretion in the absence of autoimmune destruction of beta cells. Hyperglycemia occurs when insulin secretory capacity is inadequate to overcome peripheral insulin resistance. Both genetic (polygenic inheritance) and environmental factors (obesity, particularly abdominal, and low physical activity) play a strong role in the occurrence of insulin resistance. The hereditary component results in significant differences in the prevalence of type 2 DM among ethnic groups (eg, type 2 DM is more common in certain populations including individuals of African American, Indigenous, Hispanic, and South Asian descent). The pathophysiologic pathways leading to insulin resistance and deficient insulin secretion are not completely understood, but it appears that an excessive release of free fatty acids by visceral adipose tissue, lipotoxicity caused by these free fatty acids, effects of several adipokines, metabolic stress, and chronic inflammation associated with obesity all play a role in the development of DM and also contribute to the cardiovascular complications of this disease. The risk of developing DM is increased with advancing age, obesity, lack of physical activity, hypertension, and dyslipidemia, as well as in women with prior gestational DM (GDM) or polycystic ovary syndrome (PCOS).
Prediabetes is diagnosed when levels of glucose or HbA1c (or both) do not meet the criteria for DM but are higher than what is considered normal. Its presence is associated with an increased risk of overt DM: The 5-year incidence is from 9% to 25% for HbA1c in the range of 5.5% to 6% and rises to 25% to 50% with HbA1c in the range of 6% to 6.5%. The incidence of progression to type 2 DM rises to 100% over a 5-year period when an individual has both IFG and HbA1c of 6.0% to 6.4%. Of note, the threshold at which experts suggest diagnoses of prediabetes and DM has been evolving over time and varies by location (similarly to lipid levels or blood pressure thresholds).
3. Other specific types of DM may be caused by:
1) Genetic defects of pancreatic beta-cell function (eg, maturity-onset diabetes of the young [MODY]: a group of autosomal dominant monogenic defects of insulin secretion that lead to DM diagnosed at a young age with negative beta cell autoantibodies [Table 6.2-2]).
2) Genetic defects of insulin action.
3) Pancreatic exocrine disorders and cystic fibrosis–related diabetes (CFRD).
4) Endocrinopathies (eg, Cushing syndrome, acromegaly, pheochromocytomas, glucagonomas, somatostatinomas).
5) Drugs (drug-induced DM; eg, glucocorticoids and posttransplant DM).
6) Viral infections (eg, congenital rubella).
7) Rare immune-mediated DM (eg, stiff man syndrome).
8) Ketosis-prone DM (also known as Flatbush DM).
9) Other genetic syndromes associated with DM (eg, Down syndrome, Klinefelter syndrome, Turner syndrome, Wolfram syndrome, and maternally inherited DM and deafness).
4. GDM (see Gestational Diabetes Mellitus) is defined by the presence of DM that is first diagnosed in the second or third trimester of pregnancy in women without preexisting DM. Women diagnosed with DM (standard diagnostic criteria) during the first trimester should be classified as having preexisting pregestational diabetes. GDM develops due to pregnancy-related elevation of hormones antagonistic to insulin, leading to insulin resistance, increased insulin requirements, and increased glucose availability for the developing fetus. These mechanisms result in an elevated risk of abnormal glucose metabolism, especially in the presence of obesity.
Clinical Features and Natural HistoryTop
1. The natural history of DM depends on the rate and extent of beta-cell dysfunction and destruction as well as insulin resistance caused by the combination of genetic and environmental factors. In type 1 DM the progression seems to depend on antibody expression (age of detection, their number and levels). The mechanism of type 2 DM is through a state of insulin resistance and beta-cell dysfunction. Initially type 2 DM can be underdiagnosed because of the lack of typical clinical symptoms. As the disease progresses, patients typically go from a stage of mild hyperglycemia (eg, prediabetes) to overt type 2 DM. Signs and symptoms are nonspecific and variable; they are associated with the type of DM and dynamics of disease progression, which tend to be much more abrupt in type 1 than in type 2. This may result in hyperglycemic crisis such as ketoacidosis or coma. If difficulties in achieving complete DM control occur, the development of chronic complications may not be fully prevented (see Complications of Diabetes, Chronic).
2. Signs and symptoms of DM: Nonspecific and variable, including polyuria (osmotic diuresis caused by glycosuria when serum glucose rises ≥11.1 mmol/L [200 mg/dL]), nocturia (urinating during the night), polydipsia (increased thirst), polyphagia (increased hunger), blurred vision, weight loss, weakness, and signs of hypovolemia (eg, decreased skin turgor, dry skin and mucous membranes, hypotension). Hyperglycemia may become particularly evident during a concurrent illness (eg, infection, myocardial infarction [MI]).
1) Type 1 DM: An acute loss of beta-cell reserve can lead to an acute onset of the disease with marked insulinopenia and hyperglycemia; in fact, in many patients with type 1 DM the degree of insulinopenia is significant enough to cause ketoacidosis at presentation.
2) Type 2 DM: In contrast, >50% of patients with type 2 DM are asymptomatic when the diagnosis is made (disease is frequently detected incidentally or on screening glucose measurements). The majority of patients with type 2 DM have obesity—most commonly abdominal-type obesity—and frequently a cluster of comorbidities that includes hypertension, nonalcoholic fatty liver disease, prior GDM, and dyslipidemia (with low serum high-density lipoprotein cholesterol [HDL-C] and high triglyceride [TG] concentrations). Insulin resistance is a key feature in type 2 DM, although it is not a pathognomonic finding for this type of DM (eg, patients with obesity and type 1 DM may have varying degrees of insulin resistance). Diabetic ketoacidosis (DKA) does not commonly occur in type 2 DM, except for type 2 DM of a long duration, and is precipitated by acute illness or use of certain drugs (eg, sodium-glucose cotransporter 2 [SGLT-2] inhibitors).
DiagnosisTop
Laboratory tests:
1) Blood glucose: Fasting plasma glucose (FPG) ≥7 mmol/L in venous blood (reference range, 3.9-5.5 mmol/L [70-99 mg/dL]) is used as a diagnostic test for DM and for monitoring glycemic control, whereas glucose levels in capillary full blood (measured using a glucometer) are used only for monitoring.
2) HbA1c reflects mean glycemia over the 3 months preceding the test. The mean glucose level in the immediate 30 days prior to testing contributes to ~50% of the result, whereas values from 90 to 120 days prior to testing account for ~10%. HbA1c is used both for the diagnosis of DM and for evaluation of metabolic control of the disease. The advantage of this test is that it can be measured at any time during the day and it is not affected by acute blood glucose level changes. When interpreting the results, consider other conditions that may affect its accuracy; if a condition results in a shorter life-span and greater proportion of younger erythrocytes (eg, hemolytic anemias), falsely low HbA1c values are likely. Red blood cell transfusion can also decrease HbA1c levels in patients with DM. In contrast, a longer erythrocyte life-span is associated with longer exposure to elevated blood glucose, hence falsely increasing HbA1c levels (eg, iron or vitamin B12 deficiency anemias). To avoid misdiagnosis of DM, HbA1c should be measured using a method certified by the NGSP and standardized to the Diabetes Control and Complications Trial (DCCT) assay.
3) A 75-g oral glucose tolerance test (OGTT) can be used for the screening or diagnosis of DM. A patient without acute illness is instructed to eat a diet with normal carbohydrate content in the days before the test. The OGTT is performed in the morning after 8 to 12 hours of fasting and includes measurement of FPG. Plasma glucose measurement is obtained 2 hours after the ingestion of 75 g of glucose in the form of a solution. A plasma glucose level ≥11.1 mmol/L (200 mg/dL) is consistent with DM, with normal plasma glucose levels at 2 hours being <7.8 mmol/L (140 mg/dL). A glucose level between 7.9 and 11.1 mmol/L implies IGT. A modified version of this test is used to diagnose GDM.
4) Urine glucose: Glycosuria is typically seen in patients with DM when the blood glucose level rises ≥11.1 mmol/L (200 mg/dL). In patients without DM, this can result from defects in renal tubular function (eg, proximal renal tubular acidosis). Measurement of urine glucose is not useful for the screening, diagnosis, or monitoring of DM treatment. However, the presence of glycosuria is an indication for blood glucose tests.
5) Fructosamine: This rarely used test demonstrates mean glycemia over the preceding 2 weeks (the half-life of albumin). Although this is not a validated test for diagnosing DM, fructosamine levels can be measured in patients in whom HbA1c is unreliable or in whom it is necessary to evaluate short-term blood glucose control (eg, pregnant women).
6) Islet cell antibodies can be used to confirm the autoimmune etiology of DM. At least 1 antibody is present in >90% of patients with type 1 DM, and the presence of antibodies defines patients with LADA. These antibodies may be detectable before the clinical onset of DM:
a) Antibodies to glutamate decarboxylase 65 (anti-GAD-65; most accurate).
b) Antibodies against tyrosine phosphatase–related proteins (IA-2, IA-2 beta).
c) Insulin autoantibodies (IAAs).
d) Beta-cell-specific zinc transporter antibody (ZnT-8).
7) Serum C-peptide level reflects endogenous insulin levels. It is decreased or undetectable in type 1 DM, elevated in early type 2 DM (when insulin resistance is the dominant mechanism and insulin secretion increases), and decreased in type 2 DM after the deterioration of beta-cell secretory capacity. Although the measurement of C-peptide levels is not systematically performed or recommended, it can be helpful for refined subtyping of adult-onset DM.
Systematic screening for type 1 DM is not recommended, because this condition is rare. However, the recent US Food and Drug Administration (FDA) approval of the monoclonal antibody teplizumab, which is able to delay the occurrence of type 1 DM in high-risk patients (ie, identified by the presence of ≥2 diabetes-linked autoantibodies and abnormal response to an oral glucose tolerance test),Evidence 1Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due to indirectness and imprecision. Herold KC, Bundy BN, Long SA, et al; Type 1 Diabetes TrialNet Study Group. An Anti-CD3 Antibody, Teplizumab, in Relatives at Risk for Type 1 Diabetes. N Engl J Med. 2019 Aug 15;381(7):603-613. doi: 10.1056/NEJMoa1902226. Epub 2019 Jun 9. Erratum in: N Engl J Med. 2020 Feb 6;382(6):586. PMID: 31180194; PMCID: PMC6776880. may suggest the interest to screen relatives of patients with type 1 DM. In contrast, type 2 DM is common, develops slowly, can be asymptomatic for a relatively long time, and can be treated at an early stage to prevent or delay its complications. Data on the clinical consequences of screening are, however, limited.Evidence 2Low Quality of Evidence (low confidence that we know true effects of the intervention). Quality of Evidence lowered due to indirectness and imprecision. Peer N, Balakrishna Y, Durao S. Screening for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2020 May 29;5(5):CD005266. doi: 10.1002/14651858.CD005266.pub2. PMID: 32470201; PMCID: PMC7259754. Accessed June 9, 2021.
As an example, in the case of asymptomatic individuals Diabetes Canada recommends screening for type 2 DM using a DM risk calculator in adults of any age or with any of the following risk factors:
1) A first-degree relative with DM.
2) High-risk populations (eg, African American, Latino, Indigenous, Pacific Islander, South Asian, or individuals with low socioeconomic status).
3) Delivery of a baby weighing >4 kg or confirmed diagnosis of GDM.
4) Hypertension (or antihypertensive treatment).
5) An HDL-C level <1.0 mmol/L (39 mg/dL) in men and <1.3 mmol/L (50 mg/dL) in women and/or TG level >1.7 mmol/L (150 mg/dL).
6) Physical inactivity.
7) PCOS.
8) HbA1c of 6.0% to 6.4% (42-47 mmol/mol), IGT, or IFG on previous testing.
9) Other clinical conditions associated with insulin resistance (eg, severe obesity, acanthosis nigricans, obstructive sleep apnea [OSA], nonalcoholic steatohepatitis).
10) A history of cardiovascular disease (CVD).
11) Smoking.
12) Use of medications associated with DM (glucocorticoids, antipsychotic, antiretroviral, antirejection drugs).
If the above criteria are not met, testing for DM should begin at the age of 40 years. FPG, HbA1c, and the 75-g OGTT are appropriate tests for screening. If results are negative, Diabetes Canada recommends repeating tests at least at 3-year intervals, with consideration of more frequent testing depending on the initial results and presence of risk factors (for details on risk prediction, visit the official website of the Framingham Heart Study). Other organizations issued similar suggestions, noting that the quality of evidence supporting the type of screening and its overall benefit is at most moderate. The American Diabetes Association (ADA) recommendations for screening are available at diabetesjournals.org.
DM screening tests in pregnant women: see Gestational Diabetes Mellitus.
Diagnostic workup in patients with hyperglycemia should not be performed during acute phases of other diseases (eg, infection or acute coronary syndrome), immediately following trauma or surgery, or during treatment with drugs that may cause elevated blood glucose levels (eg, glucocorticoids, thiazide diuretics, certain beta-blockers).
According to Diabetes Canada and the ADA, the diagnosis of DM is established when either of these criteria is met:
1) There are typical signs and symptoms of hyperglycemia (eg, polydipsia, polyuria, polyphagia, weight loss, blurry vision, weakness) or hyperglycemic crisis and a random plasma glucose level ≥11.1 mmol/L (200 mg/dL).
2) HbA1c ≥6.5% (48 mmol/mol) (measured using a certified method), FPG ≥7.0 mmol/L (126 mg/dL) (fasting is defined as no caloric intake for ≥8 hours), or 2-hour plasma glucose ≥11.1 mmol/L (200 mg/dL) during a 75-g OGTT. In the absence of unequivocal signs and symptoms of hyperglycemia, one abnormal test result should be confirmed by repeating the same test at a later date. If 2 different tests are available (eg, FPG and HbA1c) and both are consistent with DM, additional testing is not needed. If results of different tests are discordant, the test that is diagnostic for DM should be repeated.
According to Diabetes Canada and the ADA, the category of increased risk of DM (prediabetes) is defined by the presence of any of the following:
1) HbA1c between 6.0% and 6.4% (42-47 mmol/mol).
2) IFG (FPG of 6.1-6.9 mmol/L [110-125 mg/dL]).
3) IGT (2-hour plasma glucose after a 75-g OGTT between 7.8-11.1 mmol/L [140-200 mg/dL]).
1. Other causes of clinical signs and symptoms, such as polyuria (diabetes insipidus).
2. Other causes of hyperglycemia: Stress-induced hyperglycemia, which refers to transient hyperglycemia and may occur during acute illness or significant stress in patients without DM (eg, sepsis, acute coronary syndrome, immediately following trauma or major surgery).
TreatmentTop
The management of DM includes:
1) Patient education, which is indispensable for treatment success.
2) Nonpharmacologic management: Nutrition, weight loss, and exercise.
3) Glucose-lowering treatment: Oral and injectable antidiabetic agents, insulin.
4) Management of other cardiovascular risk factors, particularly, hypertension (see Essential Hypertension), dyslipidemia (see Hypercholesterolemia), smoking (see Nicotine Addiction), and obesity (see Obesity: General Considerations). Control of these risk factors is of utmost importance for preventing morbidity and mortality in patients with DM.
5) Prevention and management of chronic diabetic complications.
1. In type 2 DM lifestyle modification, dietary changes, physical activity, and weight loss are the fundamental aspects of care. An intensive behavioral lifestyle intervention program should be suggested to all patients with type 2 DM, including those with prediabetes, in order to induce and maintain a loss of ~7% to 10% of initial body weight and to increase moderate-intensity physical activity to ≥150 min/wk. In patients with prediabetes this program has been shown to reduce the incidence of type 2 DM by 58% over 3 years. Of note, patients’ willingness and ability to conform to recommendations concerning lifestyle modifications vary widely and cannot be assumed or even expected.
In certain cases bariatric surgery should be recommended to patients with type 2 DM and obesity with body mass index (BMI) >30.0 kg/m2 when lifestyle interventions with or without weight management medications are not adequate in achieving glycemic targets. Bariatric surgery frequently leads to remission of type 2 DM and delays the progression of albuminuria.
Over the last few years, glucagon-like peptide-1 (GLP-1) receptor agonists liraglutide and semaglutide have been increasingly used not only for DM treatment but also for weight loss, with expectations of improving glycemic control and reducing risk factors for cardiometabolic disease (see below; also see Obesity: General Considerations). Even more recently tirzepatide, a novel glucose-dependent insulinotropic polypeptide (GIP) and GLP-1 receptor agonist (already approved for type 2 DM management), has shown significant reduction in body weight in people with obesity, both with and without DM,Evidence 3Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due to indirectness and imprecision. Jastreboff AM, Aronne LJ, Ahmad NN, et al; SURMOUNT-1 Investigators. Tirzepatide Once Weekly for the Treatment of Obesity. N Engl J Med. 2022 Jul 21;387(3):205-216. doi: 10.1056/NEJMoa2206038. Epub 2022 Jun 4. PMID: 35658024. and is expected to be approved by regulatory authorities for weight management in 2023.
1) In type 1 DM insulin therapy is mandatory once the diagnosis of DM is established, dispensed through multiple daily subcutaneous injections or a pump.
2) In type 2 DM insulin therapy is indicated in patients not achieving appropriate glycemic control with other medications. Insulin should also be started in patients with type 2 DM and marked hyperglycemia at the time of diagnosis (eg, HbA1c >11% [97 mmol/mol]) and in patients with hyperglycemic crisis (ie, DKA, hyperosmolar hyperglycemic state [HHS]). If insulin treatment is used early in the course of DM because of beta-cell glucotoxicity, recovery of beta-cell function after achieving adequate control of hyperglycemia may allow for de-escalation of insulin therapy and often switching to oral or injectable antidiabetic medications. As type 2 DM is a progressive disease with a gradual deterioration of the secretory capacity of pancreatic beta cells, many patients with type 2 DM eventually need insulin therapy.
3. In type 2 DM metformin has traditionally been recommended as first-line glucose-lowering therapy. However, there is ongoing acceptance that other approaches may be appropriate. Notably, the benefits of GLP-1 receptor agonists and SGLT-2 inhibitors for cardiovascular and renal outcomes have been found to be independent of metformin use, and thus these agents should be considered in people with established or high risk of CVD, heart failure (HF), or chronic kidney disease (CKD), independent of metformin use (Figure 6.2-4). Early combination therapy based on the need for additional glycemic efficacy or cardiorenal protection can be considered at treatment initiation. Monotherapy with SGLT-2 inhibitors or GLP-1 receptor agonists initiated instead of metformin, if indicated by the presence of HF, CKD, or atherosclerotic cardiovascular disease (ASCVD), can be an option. Different glucose-lowering medications are currently available, including insulin secretagogues (eg, mostly sulfonylureas and meglitinides, incretins [dipeptidyl peptidase-4 (DPP-4) inhibitors] and GLP-1 receptor agonists), insulin sensitizers (eg, metformin, thiazolidinediones [TZDs]), alpha-glucosidase inhibitors (eg, acarbose), and SGLT-2 inhibitors (eg, canagliflozin or empagliflozin). As recent cardiovascular and renal outcome trials of noninsulin agents, particularly SGLT-2 inhibitors and GLP-1 receptor agonists, have shown significant cardiorenal benefits, it is important to consider the presence of cardiovascular or renal risk factors, ASCVD, and history of HF or CKD in patients prior to choosing an agent for additional benefits beyond glycemic control.Evidence 4Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of the intervention). Palmer SC, Tendal B, Mustafa RA, et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ. 2021 Jan 13;372:m4573. doi: 10.1136/bmj.m4573. PMID: 33441402; PMCID: PMC7804890. Li S, Vandvik PO, Lytvyn L, et al. SGLT-2 inhibitors or GLP-1 receptor agonists for adults with type 2 diabetes: a clinical practice guideline. BMJ. 2021 May 11;373:n1091. doi: 10.1136/bmj.n1091. PMID: 33975892.
4. If the type of DM is unclear (ie, type 1 vs type 2) in a patient presenting with hyperglycemic crisis, the final diagnosis and appropriate long-term treatment can be established after control of metabolic abnormalities is achieved with insulin therapy. If autoimmune etiology of DM is excluded, patients can be sometimes successfully switched to oral or injectable noninsulin glucose-lowering medications.
5. Target HbA1c levels should be achieved gradually (ie, over several weeks to months) because a rapid reduction of plasma glucose levels carries a risk of hypoglycemia (particularly in type 1 DM), and in patients with advanced microangiopathy (primarily retinopathy) it may accelerate the progression of this complication. In patients who do not achieve target HbA1c levels despite maintaining target FPG, make attempts to reduce postprandial glucose levels, especially if HbA1c levels are close to target.
6. Criteria of DM control: Glycemic goal: The intensity of glucose-lowering treatment, determined by target blood glucose and HbA1c values, should be individualized based on the patient’s cooperation and motivation, risk of hypoglycemia, disease duration, life expectancy, comorbidities, cardiovascular complications, frailty, as well as financial resources and support available. The criteria of DM control may be less stringent in the elderly, in patients with comorbidities, and in those with frequent episodes of hypoglycemia. If target values cannot be achieved, attempts should be made to achieve results as close as practically possible. Of note, different professional societies recommend different targets, from 6.5% (American Association of Clinical Endocrinologists) through 7% (ADA) to between 7% and 8% (American College of Physicians). This changes with time and may make clinicians less anxious about rigid adherence to specific values.Evidence 5High Quality of Evidence (high confidence that we know true effects of the intervention). Abbasi J. For Patients With Type 2 Diabetes, What's the Best Target Hemoglobin A1C? JAMA. 2018 Jun 19;319(23):2367-2369. doi: 10.1001/jama.2018.5420. PubMed PMID: 29847622. The current Diabetes Canada guidelines suggest a range of values: from ≤6.5% in adults with DM if they are at low risk of hypoglycemia, especially in the case of recent-onset DM; through ≤7% in most adults; and up to 8.5% in those functionally dependent, with recurrent hypoglycemia or hypoglycemia unawareness, limited life expectancy, frailty, or cognitive impairment (dementia).
The ADA suggests:
1) Target HbA1c levels <7.0% (53 mmol/mol) and preprandial capillary blood glucose levels between 3.9 and 7.2 mmol/L (70-130 mg/dL) in most nonpregnant adults with DM. To achieve this in young patients with type 1 DM, multiple daily injection insulin therapy is usually required, sometimes the use of an insulin pump. ADA experts acknowledge that individual patients’ goals may be slightly lower or slightly higher. We consider the advice to achieve this target as a strong recommendation in type 1 DMEvidence 6Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). Moderate Quality of Evidence (moderate confidence that we know true effects of intervention). Quality of Evidence lowered due to heterogeneity of effects in individual patients. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993 Sep 30;329(14):977-86. PMID: 8366922. Nathan DM, Cleary PA, Backlund JY, et al; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005 Dec 22;353(25):2643-53. PMID: 16371630; PMCID: PMC2637991. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998 Sep 12;352(9131):837-53. Erratum in: Lancet 1999 Aug 14;354(9178):602. PMID: 9742976. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998 Sep 12;352(9131):837-53. Erratum in: Lancet 1999 Aug 14;354(9178):602. PMID: 9742976. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008 Oct 9;359(15):1577-89. doi: 10.1056/NEJMoa0806470. Epub 2008 Sep 10. PMID: 18784090. ADVANCE Collaborative Group, Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008 Jun 12;358(24):2560-72. doi: 10.1056/NEJMoa0802987. Epub 2008 Jun 6. PMID: 18539916. Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009 Jan 8;360(2):129-39. doi: 10.1056/NEJMoa0808431. Epub 2008 Dec 17. Erratum in: N Engl J Med. 2009 Sep 3;361(10):1028. N Engl J Med. 2009 Sep 3;361(10):1024-5. PMID: 19092145. Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008 Jun 12;358(24):2545-59. doi: 10.1056/NEJMoa0802743. Epub 2008 Jun 6. PMID: 18539917; PMCID: PMC4551392. and a weak suggestion in type 2 DM.Evidence 7Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). Moderate Quality of Evidence (moderate confidence that we know true effects of intervention). Quality of Evidence lowered due to heterogeneity of effects in individual patients. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993 Sep 30;329(14):977-86. PMID: 8366922. Nathan DM, Cleary PA, Backlund JY, et al; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005 Dec 22;353(25):2643-53. PMID: 16371630; PMCID: PMC2637991. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998 Sep 12;352(9131):837-53. Erratum in: Lancet 1999 Aug 14;354(9178):602. PMID: 9742976. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998 Sep 12;352(9131):837-53. Erratum in: Lancet 1999 Aug 14;354(9178):602. PMID: 9742976. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008 Oct 9;359(15):1577-89. doi: 10.1056/NEJMoa0806470. Epub 2008 Sep 10. PMID: 18784090. ADVANCE Collaborative Group, Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008 Jun 12;358(24):2560-72. doi: 10.1056/NEJMoa0802987. Epub 2008 Jun 6. PMID: 18539916. Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009 Jan 8;360(2):129-39. doi: 10.1056/NEJMoa0808431. Epub 2008 Dec 17. Erratum in: N Engl J Med. 2009 Sep 3;361(10):1028. N Engl J Med. 2009 Sep 3;361(10):1024-5. PMID: 19092145. Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008 Jun 12;358(24):2545-59. doi: 10.1056/NEJMoa0802743. Epub 2008 Jun 6. PMID: 18539917; PMCID: PMC4551392.
2) HbA1c levels <6.5% (48 mmol/mol) are suggested for selected patients with a short duration of DM, contemplating pregnancy, long life expectancy, and no significant CVD, as long as treatment does not induce significant hypoglycemia.
3) In contrast, the criteria of DM control may be less stringent (HbA1c <8.0% [64 mmol/mol]) in the elderly, in patients with significant comorbidities, advanced microvascular or macrovascular complications, limited life expectancy, and in patients who developed hypoglycemia unawareness or those with severe or frequent episodes of hypoglycemia.
4) Considering the lack of clear benefits on major outcomes, risk of hypoglycemia, and potential burden and higher costs of more intensive treatment, a strong recommendation against intensive glycemic control (eg, HbA1c ≤6.5% [48 mmol/mol]) can be made for older patients with long-standing type 2 DM and risk for CVD or hypoglycemia.Evidence 8Strong recommendation (downsides clearly outweigh benefits; right action for all or almost all patients). Moderate Quality of Evidence (moderate confidence that we know true effects of intervention). Quality of Evidence lowered due to indirectness of evidence to that particular population. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993 Sep 30;329(14):977-86. PMID: 8366922. Nathan DM, Cleary PA, Backlund JY, et al; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005 Dec 22;353(25):2643-53. PMID: 16371630; PMCID: PMC2637991. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998 Sep 12;352(9131):837-53. Erratum in: Lancet 1999 Aug 14;354(9178):602. PMID: 9742976. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998 Sep 12;352(9131):837-53. Erratum in: Lancet 1999 Aug 14;354(9178):602. PMID: 9742976. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008 Oct 9;359(15):1577-89. doi: 10.1056/NEJMoa0806470. Epub 2008 Sep 10. PMID: 18784090. ADVANCE Collaborative Group, Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008 Jun 12;358(24):2560-72. doi: 10.1056/NEJMoa0802987. Epub 2008 Jun 6. PMID: 18539916. Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009 Jan 8;360(2):129-39. doi: 10.1056/NEJMoa0808431. Epub 2008 Dec 17. Erratum in: N Engl J Med. 2009 Sep 3;361(10):1028. N Engl J Med. 2009 Sep 3;361(10):1024-5. PMID: 19092145. Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008 Jun 12;358(24):2545-59. doi: 10.1056/NEJMoa0802743. Epub 2008 Jun 6. PMID: 18539917; PMCID: PMC4551392.
5) The role of postprandial blood glucose targets is especially important in individuals with an HbA1c level that is close to a target of ≤7.0%, while FPG is the major contributor with higher HbA1c levels. According to Diabetes Canada, postprandial testing aiming at blood glucose values <10 mmol/L (180 mg/dL) 1 to 2 hours after the beginning of a meal is a reasonable strategy in patients with high HbA1c and preprandial glucose levels within target values.
6) According to the Fifth International Workshop-Conference on Gestational Diabetes Mellitus, the following target values should be used for capillary glucose concentrations in pregnant patients: preprandial level, ≤5.3 mmol/L (95 mg/dL); 1-hour postprandial level ≤7.8 mmol/L (140 mg/dL) and/or 2-hour postprandial level ≤6.7 mmol/L (120 mg/dL). For patients with preexisting type 1 or type 2 DM who become pregnant, the optimal recommended glycemic goals are as follows, provided they can be achieved without excessive hypoglycemia:
a) Preprandial, bedtime, and overnight glucose: 3.3 to 5.3 mmol/L (60-95 mg/dL).
b) Peak 1-hour postprandial glucose: ≤7.8 mmol/L (140 mg/dL) and/or 2-hour postprandial level ≤6.7 mmol/L (120 mg/dL).
c) HbA1c: <6.1% (43 mmol/mol), if it can be safely achieved.
Given the development of continuous glucose monitoring (CGM) (see Continuous Glucose Monitoring, below), optimal glycemic control is evaluated based on not only HbA1c targets but also glycemic targets as assessed by time in range (TIR), which is defined by a blood glucose level between 3.9 and 10.0 mmol/L (70-180 mg/dL).
7. Principles of lipid control: In patients with diabetes, Diabetes Canada recommends targeting low-density lipoprotein cholesterol (LDL-C) <2.0 mmol/L or a >50% reduction from baseline. Alternative targets and goals that are recommended include non–HDL-C <2.6 mmol/L or apolipoprotein B <0.8 g/L.
Diabetes Canada recommendations include:
1) Intensifying lifestyle therapy and optimizing glycemic control for patients with TG levels ≥1.7 mmol/L (150 mg/dL) and/or HDL-C <1.0 mmol/L (40 mg/dL) for men or <1.3 mmol/L (50 mg/dL) for women.
2) For patients with TG levels ≥5.7 mmol/L (500 mg/dL), it is important to exclude secondary causes and consider medical therapy (eg, fibrates) to reduce the risk of pancreatitis, especially with TG >10 mmol/L.
3) Statin therapy should be used to reduce cardiovascular risk in adults with type 1 or type 2 DM with any of the following:
a) Clinical CVD.
b) Age ≥40 years.
c) Age <40 years and 1 of the following: DM duration >15 years and age >30 years; microvascular complications; cardiovascular risk.
4) Patients who do not tolerate statin therapy due to adverse effects should be maintained on a maximal tolerated dose. Consider adding ezetimibe to a maximal tolerated statin dose if LDL-C is not at target.
5) Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors can be added to statin therapy when LDL-C targets are not met in patients with ASCVD or familial hypercholesterolemia.
6) For patients with ASCVD with well-controlled LDL-C levels but elevated fasting TGs between 1.52 and 5.6 mmol/L (135-499 mg/dL), consider adding icosapent ethyl (IPE) to further reduce cardiovascular risk.
8. Principles and criteria of blood pressure control: According to Diabetes Canada, patients with DM should be treated to achieve a blood pressure target of <130/80 mm Hg.
According to the ADA 2023 and Diabetes Canada guidelines:
1) A general recommendation for starting a single antihypertensive agent in patients with DM includes a blood pressure ≥130/80 mm Hg, and for starting 2 agents (preferably as a single-pill combination), blood pressure ≥150/90 mm Hg.
2) Angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin-receptor blockers (ARBs) are frequently suggested as first-line antihypertensive agents, particularly among patients with evidence of diabetic nephropathy.Evidence 9Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). Moderate Quality of Evidence (moderate confidence that we know true effects of intervention). Quality of Evidence lowered due to heterogeneity of risks, benefits, and adverse effects in individual patients. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014 Feb 5;311(5):507-20. doi: 10.1001/jama.2013.284427. Erratum in: JAMA. 2014 May 7;311(17):1809. PMID: 24352797. Arguedas JA, Leiva V, Wright JM. Blood pressure targets for hypertension in people with diabetes mellitus. Cochrane Database Syst Rev. 2013 Oct 30;10:CD008277. doi: 10.1002/14651858.CD008277.pub2. Review. PubMed PMID: 24170669. Wu HY, Huang JW, Lin HJ, et al. Comparative effectiveness of renin-angiotensin system blockers and other antihypertensive drugs in patients with diabetes: systematic review and bayesian network meta-analysis. BMJ. 2013 Oct 24;347:f6008. doi: 10.1136/bmj.f6008. Review. PMID: 24157497; PMCID: PMC3807847. Cheng J, Zhang W, Zhang X, et al. Effect of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on all-cause mortality, cardiovascular deaths, and cardiovascular events in patients with diabetes mellitus: a meta-analysis. JAMA Intern Med. 2014 May;174(5):773-85. doi: 10.1001/jamainternmed.2014.348. PMID: 24687000. Second-line agents include dihydropyridine calcium channel blockers (DHP-CCBs) or thiazide or thiazide-like diuretics.
Recent studies have shown that SGLT-2 inhibitors provide cardiorenal protection associated with cardiac benefits and delays in the progression of albuminuria or CKD in high-risk patients with cardiac and renal diseases with or without DM. Neither the ADA nor Diabetes Canada have modified their guidelines on blood pressure in patients with DM to reflect this change; however, it should be considered in practice.
1. Patient education is an important component of DM management, together with nutrition therapy, exercise, and pharmacotherapy. It should be offered to all patients.Evidence 10Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). Moderate Quality of Evidence (moderate confidence that we know true effects of intervention). Quality of Evidence lowered due to uncertainty of the effects of individual components. Deakin T, McShane CE, Cade JE, Williams RD. Group based training for self-management strategies in people with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2005 Apr 18;(2):CD003417. Review. Update in: Cochrane Database Syst Rev. 2015;6:CD003417. PMID: 15846663. Duke SA, Colagiuri S, Colagiuri R. Individual patient education for people with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2009 Jan 21;(1):CD005268. doi: 10.1002/14651858.CD005268.pub2. Review. PMID: 19160249.
2. Patient education aims to improve knowledge, skills, and confidence in DM management and to promote the patient’s cooperation with a multidisciplinary therapeutic team. The reinforcement for DM self-management education must be addressed at diagnosis, annually, in case of appearance of new complicating factors, and when transitions in care occur.
3. Education programs typically cover aspects of the pathophysiology of DM, lifestyle modification, glucose self-monitoring, insulin dose adjustment, hypoglycemia management, prevention and detection of acute and chronic DM complications, and foot care. Additionally, health status and quality of life evaluation is also included.
4. Educational sessions should be patient-centered and repeated and their effects should be evaluated, including not only the patients’ knowledge but also their capability of coping with the disease and empowerment to make informed self-management decisions. The inclusion of patient-centered care must be respectful of and responsive to individual patient preferences, needs, and values. Structured education programs that promote intensive basal-bolus insulin therapy and teach the principles of dose-adjustment have been associated with improvements in glycemic control and quality of life in patients with type 1 DM. In patients with type 2 DM education should include teaching about the likely progressive nature of the disease and the necessary gradual modifications of treatment.
5. Patient education can be optimally conducted both in individual and group settings. This should include coping strategies as well as resources, including access to psychotherapy, psychologists, social workers, and psychiatrists, to address any mental health challenges faced when living with a chronic disease such as DM.
Self-Monitoring of Blood Glucose
All patients with DM who use insulin or take other glucose-lowering medications that can cause hypoglycemia (eg, sulfonylureas) should learn how to check their finger-stick capillary blood glucose with a glucose meter. The recommended frequency of self-monitoring of blood glucose (SMBG) depends on the type of antidiabetic therapy and long-term stability of clinical status. SMBG is a fundamental aspect of management in type 1 DM and is also important in patients with type 2 DM treated with complex insulin regimens. Both the ADA and Diabetes Canada suggest that patients treated with multiple-dose insulin or insulin pump therapy should consider SMBG prior to meals and snacks, postprandially, at bedtime, prior to exercise, when hypoglycemia is suspected, after treating hypoglycemia, and prior to critical tasks such as driving or other high-risk tasks (eg, operating machinery). For some patients it may mean ≥6 measurements per day. Patients with type 2 DM treated with oral agents that can cause hypoglycemia also likely benefit from SMBG, particularly during uptitration of these medications (eg, testing once to twice daily before breakfast and before the evening meal).
In contrast, the benefit of SMBG in patients with type 2 DM on diet only or those who are treated with medications not associated with hypoglycemia is controversial, although it may have an effect on behavior, diet change, or both. The ADA suggests that SMBG results may be helpful to guide treatment decisions in patients treated with noninsulin therapies. In this context a reasonable frequency of measurements will depend on the patient’s preference. Motivated patients with type 2 DM could take action to modify diet or exercise patterns based on SMBG readings and therefore improve their HbA1c values.
CGM devices measure the interstitial glucose concentration in real time and display the level on the reader or compatible mobile devices via Bluetooth. The sensor, which can be worn for up to 14 days, captures and relays 24-hour data and glucose trends along with alarms for high, low, or rapid changes in glucose levels. Real-time CGM (rtCGM) systems automatically display the glucose value. Intermittently scanned CGM (isCGM) systems require the person using the system to scan the sensor to display glucose information. These devices can be used independently or paired with insulin pumps. Studies have shown a significant reduction in time spent in hypoglycemia, increased TIR, and overall improvement in glycemic control (HbA1c) and variability.
Medical Nutrition Therapy: General Considerations
The ADA recommends nutrition therapy for all patients with type 1 and type 2 DM. Nutrition therapy consists of the development of eating patterns designed to achieve and maintain an ideal body weight, improve glycemic control, lower blood pressure, improve lipid profile, reduce cardiovascular risk, and reduce the overall risk for both acute and long-term complications of DM while preserving the pleasure of eating. Nutrition therapy should aim for a beneficial effect in the overall health of patients while taking into consideration their personal and cultural preferences as well as their individual nutritional needs and their ability to sustain recommendations in the plan.
1. Adequate caloric intake should ensure maintaining an ideal body weight or gradual reduction of body weight in patients with obesity or overweight. A weight loss ≥5% in patients with type 2 DM is needed in order to produce beneficial outcomes in glycemic control, lipids, and blood pressure.
2. An optimal body weight is usually a BMI between 18.5 and 24.9 kg/m2. Healthy weight-loss diets typically aim to achieve an energy deficit of 500 to 750 kcal/d or reduce daily energy intake to 1200 to 1500 kcal in women and 1500 to 1800 kcal in men, depending on the initial weight (eg, in women >135 kg start with 1600 kcal/d, in men >135 kg start with 1800 kcal/d). Diets <1200 kcal/d for women or <1500 kcal/d in men are not generally recommended because they may be deficient in nutrients. Furthermore, very low-calorie diets have not been found to produce greater long-term weight losses than conventional low-calorie diets. The Mediterranean diet, structured low-calorie meal plan, low-fat eating plan, plant-based diet, or Dietary Approaches to Stop Hypertension (DASH) meal plan are the ones most suggested for patients with prediabetes and DM. Low-carbohydrate diets have been shown to improve hyperglycemia, reduce HbA1c, and reduce the need for antihyperglycemic medications in some patients with type 2 DM. Overall, lifestyle modifications, which include dietary changes, are recommended.Evidence 11Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). Moderate Quality of Evidence (moderate confidence that we know true effects of intervention). Quality of Evidence lowered due to some heterogeneity among patient-important outcomes. Nield L, Moore HJ, Hooper L, et al. Dietary advice for treatment of type 2 diabetes mellitus in adults. Cochrane Database Syst Rev. 2007 Jul 18;(3):CD004097. Review. PMID: 17636747. Look AHEAD Research Group, Wing RR, Bolin P, Brancati FL, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013 Jul 11;369(2):145-54. doi: 10.1056/NEJMoa1212914. Epub 2013 Jun 24. Erratum in: N Engl J Med. 2014 May 8;370(19):1866. PMID: 23796131; PMCID: PMC3791615.
3. The optimal distribution of calories from carbohydrates, protein, and fat to facilitate weight loss is unknown and likely not absolute. Macronutrient distribution should be based on an individual assessment of current eating patterns, preferences, and metabolic goals.
4. Carbohydrate intake is the most important determinant of postprandial glucose levels in patients with DM. The ADA suggests choosing nutrient-dense carbohydrates containing vitamins, minerals, and fiber (eg, vegetables, whole grains, legumes, or fruit) with a low glycemic index and load over processed carbohydrates high in calories, sugar, sodium, and fat. Avoiding sugar-sweetened beverages and processed “low-fat” or “nonfat” food products with high amounts of refined grains and added sugars is also recommended. A minimum of 14 g of fiber per 1000 kcal should be consumed daily. In patients with type 2 DM taking insulin secretagogues (eg, sulfonylureas) or insulin, meals should include carbohydrates to reduce the risk of hypoglycemia.
5. Protein intake recommendations are the same as for the general population (1-1.5 g/kg of body weight per day or 15%-20% of total calories). It can be increased to 20% to 30% of total calories to increase satiety in some patients, based on an individual approach. A reduction by <0.8 g/kg of body weight per day is not recommended as it has not been shown to improve glycemic control or rate of glomerular filtration rate (GFR) decline in patients with diabetic kidney disease (DKD).
6. Fat quality is more important than quantity for reducing the risk of CVD. An acceptable macronutrient distribution for total fat is generally 20% to 35% of total calorie intake. The ADA suggests limiting the intake of saturated fat to 10% of calories, limiting the intake of cholesterol to <300 mg/d, and avoiding trans-fat as much as possible. These recommendations apply to the general population. It is also suggested to limit sodium intake to <2300 mg/d.
7. In patients treated with metformin, periodic testing of vitamin B12 levels may be considered, especially in those with anemia and peripheral neuropathy. There is lack of evidence with regards to efficacy of routine supplementation with antioxidants (vitamins E and C, carotene), herbals, and micronutrients (cinnamon, curcumin, vitamin D, chromium). Therefore, their use should not be recommended, except for special populations (pregnant or lactating women, older adults, vegetarians, and people with very low-calorie or low-carbohydrate diets).
8. Excessive alcohol intake is associated with hypoglycemia, weight gain, and hyperglycemia. Moderate alcohol intake (up to 2 drinks per day for men, 1 drink per day for women) is not associated with detrimental effects on glycemic control.
Dietary Considerations With Insulin Therapy
1. For patients with type 2 DM (or type 1 DM) treated with fixed doses of short-acting or rapid-acting and intermediate-acting insulin (frequently premixed), day-to-day consistency in the time of insulin administration, mealtimes, and amount of carbohydrate intake is an important consideration in order to avoid variable and unpredictable blood glucose levels and hypoglycemia. These patients should not skip meals.
2. For patients with type 1 DM (or type 2 DM) following a multiple daily injection program treated with a long-acting insulin and fixed doses of a rapid-acting prandial insulin, it is important to eat similar amounts of carbohydrates during each meal to match the prandial insulin doses. This program gives more flexibility regarding the time when meals can be consumed. Different meal planning strategies can be used to quantify carbohydrate intake (eg, sample menus, the exchange system [list of servings in 6 categories that may be exchanged for one another containing a similar amount of main nutrients], or carbohydrate counting). The ADA recommends the carbohydrate-counting approach for patients with type 1 DM on a flexible multiple daily injection program. Patients using insulin pumps also need to learn carbohydrate counting.
1. Diabetes Canada, the ADA, and the American Heart Association (AHA) recommend performing ≥150 minutes of moderate intensity aerobic physical activity (eg, brisk walking) per week. Physical activity should be distributed over ≥3 days per week, with no more than 2 consecutive days without activity, and should be supplemented by increase in daily lifestyle activities (eg, gardening, household work). The exercise regimen should also include resistance training. At least 90 minutes of vigorous aerobic exercise per week is an alternative. For long-term maintenance of a major weight loss, the ADA and AHA recommend a larger amount of exercise (eg, 7 hours of moderate or vigorous aerobic physical activity per week). Special considerations should be addressed in patients with CVD, uncontrolled retinopathy or nephropathy, and severe neuropathy.
2. Exercise can improve glycemic control, assist with weight loss and maintenance, and positively affect different cardiovascular risk factors, including hypertension and dyslipidemia. Resistance training (eg, exercise with elastic bands or weight machines) may confer additional benefits, as it has the potential to enhance skeletal muscle mass and improve muscle strength and insulin sensitivity.
3. Patients with significant hyperglycemia (eg, blood glucose ≥13.9 mmol/L [250 mg/dL]) should avoid vigorous exercise because they may experience worsening of hyperglycemia and ketosis. Other occasional complications associated with strenuous physical activity include foot-stress fractures, retinal bleeding in patients with proliferative retinopathy (particularly during resistance training), and acute coronary events.
4. Although many individuals with DM do not need exercise stress testing before undertaking exercise more intense than brisk walking, pre-exercise evaluation and exercise stress testing should be considered in those at high risk of CVD (eg, multiple cardiovascular risk factors, known coronary artery disease, cerebrovascular disease, or peripheral artery disease), advanced nephropathy with renal failure, or cardiovascular autonomic neuropathy.
5. Patients receiving insulin treatment should measure their blood glucose before, during, and after exercise to identify glycemic patterns that can be used to develop strategies to avoid hypoglycemia. Ideally, exercise should be performed at similar times and in a consistent relation to meals and insulin injections. Some strategies to prevent hypoglycemia include consuming extra carbohydrates before exercise and then at 30-minute intervals during exercise (eg, 15-30 g of quickly absorbed carbohydrates) as well as after the end of exercise if it was prolonged; this is particularly important in type 1 DM. In type 2 DM the risk of hypoglycemia is lower; patients with obesity usually do not need extra carbohydrates during exercise.
6. Avoid insulin injections in body areas that are especially active during a particular activity (eg, thigh) and reduce the dose of insulin that affects the time when exercise will be performed (eg, by 30%-50%), depending on exercise intensity and glucose levels.
The term human insulin denotes genetically human insulin produced by Escherichia coli (examples: Humulin R; neutral protamine Hagedorn [NPH] insulin, also known as isophane insulin).
For a major proportion of patients treated with insulin, the advantages of using insulin analogues (modified human insulin, Table 6.2-3) over human insulin include reduction in hypoglycemia with their longer duration of action and more flexibility despite the cost being 2 to 10 times higher.Evidence 12Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Hemmingsen B, Metzendorf M-I, Richter B. (Ultra‐)long‐acting insulin analogues for people with type 1 diabetes mellitus. Cochrane Database Syst Rev. 2021 Mar 4;3(3):CD013498. doi: 10.1002/14651858.CD013498.pub2. PMID: 33662147; PMCID: PMC8094220. Fullerton B, Siebenhofer A, Jeitler K, et al. Short‐acting insulin analogues versus regular human insulin for adult, non‐pregnant persons with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2018 Dec 17;12(12):CD013228. doi: 10.1002/14651858.CD013228. PMID: 30556900; PMCID: PMC6517032. Accessed June 10, 2021.
1. Indications for insulin therapy:
1) Type 1 DM: All patients with type 1 DM should be treated with insulin from the moment of diagnosis (LADA may be an exception). These patients should not stop their basal insulin administration, even during fasting.
2) Type 2 DM:
a) Patients presenting with an acute DM complication or with significant hyperglycemia at the time of diagnosis (eg, DKA, HHS, FPG >16.7 mmol/L [300 mg/dL], HbA1c >11% [97 mmol/mol], or signs of increased catabolism such as weight loss, polyuria, and polydipsia). The requirement for insulin may be temporary.
b) Failure of noninsulin antidiabetic treatment despite intensification of pharmacotherapy as well as lifestyle and behavioral interventions. In these patients insulin therapy should not be delayed. Insulin regimens can be combined with other noninsulin antidiabetic medications. Combined GLP-1 receptor agonists and insulin can be considered if HbA1c is >10% (86 mmol/mol) and/or >2% (23 mmol/mol) above the glycemic goal.
c) Insulin may be needed in hospitalized patients, as it allows for greater flexibility and safety in the management of hyperglycemia during acute illness.
2. Types of insulin: Table 6.2-3. The selection of insulin preparations and insulin regimen should be individualized to the patient’s lifestyle, usual mealtimes, and preferences.
1) Basal insulin preparations:
a) Intermediate-acting human insulin (insulin isophane [NPH]) is administered subcutaneously once or twice daily (typically in the morning before breakfast and before the evening meal or at bedtime). It is frequently given in combination with a short-acting insulin.
b) Long-acting basal insulin analogues are usually administered subcutaneously once daily, in the morning or evening, at a fixed time. However, the effect of insulin detemir can last <24 hours, and therefore bid administration is frequently required with this basal insulin (in the morning and evening). In occasional situations insulin glargine also requires twice-daily dosing (eg, early morning hyperglycemia in patients taking insulin glargine before breakfast who also experience hypoglycemia while fasting during the day, patients susceptible to hypoglycemia while on very low total daily doses of insulin, or patients using very high basal insulin doses). Long-acting analogues are frequently used in combination with rapid-acting insulin analogues as part of an intensive insulin therapy regimen (Figure 6.2-1).
Some newer insulin analogue formulations are more concentrated and act >24 hours. These include glargine (300 IU/mL) and degludec (200 IU/mL), which contain 3 and 2 times more units of insulin per unit volume, respectively, than the basic preparations (100 IU/mL), provide greater stability of glycemia given the longer time of action, and decrease the risk of nocturnal hypoglycemia.
2) Prandial insulin preparations:
a) Rapid-acting insulin analogues are administered subcutaneously immediately (within 0-15 minutes) before a meal, although they should be administered before or at start of a meal, usually 3 times a day.
b) Short-acting human insulin (regular insulin) is administered subcutaneously within 30 to 45 minutes before meals, usually 3 times a day. It is commonly administered together with an intermediate-acting insulin (Figure 6.2-2).
3) Premixed insulin preparations (insulin combinations, biphasic insulins):
a) Premixed human insulins: A short-acting human insulin combined with an intermediate-acting insulin.
b) Premixed insulin analogues: A rapid-acting insulin analogue combined with a long-acting protamine suspension of this analogue.
With premixed insulin preparations the proportion of short-acting to long-acting insulin is fixed. Depending on preparation, 50% to 80% of the insulin dose is given as an intermediate-acting or long-acting form of insulin (Table 6.2-4). Each insulin preparation in a combination product achieves its peak activity at a different time. The peaks associated with the effect of rapid-acting insulin or short-acting insulin are higher and their duration is shorter than those associated with intermediate-acting or long-acting insulins. These premixed insulin preparations are typically administered as 2 daily doses, before breakfast and before the evening meal (Figure 6.2-3). Patients must consume a meal after each injection and should follow a diet consistent in carbohydrates from day to day, with meals consumed at similar times of the day. Because of the fixed ratios of insulins, individual basal and prandial dose adjustments cannot be made. Premixed insulin preparations should ideally be used after basal insulin requirements have been first established.
3. Initial insulin doses: Most patients with type 1 DM are sensitive to insulin. It is recommended to start with a dose of 0.5 IU/kg/d. However, patients with type 1 DM may require a total daily insulin dose that ranges from 0.4 to 1.0 IU/kg/d. In type 1 DM insulin regimens typically try to mimic the physiologic release of insulin by administering a basal form of insulin (eg, glargine, detemir, or degludec) and mealtime (prandial) boluses of short-acting or rapid-acting insulin. As an initial strategy, half of the total daily insulin dose can be administered as basal (eg, 0.25 IU/kg) and half as the daily prandial dose (eg, 0.25 IU/kg divided into 3 doses given with each main meal).
In contrast, in type 2 DM it should be considered whether the patient requires full doses of insulin (eg, 0.3-0.6 IU/kg/d) or low doses of intermediate-acting or long-acting insulin added to other glucose-lowering medications (10 or 0.1-0.2 IU/kg/d of a long-acting or intermediate-acting insulin), to achieve appropriate glycemic control. When full doses of insulin are required (0.3-0.6 IU/kg/d), patients who are sensitive to insulin or predisposed to complications of hypoglycemia (eg, thin, elderly, those with adrenal insufficiency, advanced kidney disease, cirrhosis, unstable coronary artery disease, active intracranial pathology, terminal illness) require lower starting insulin doses than those who are more insulin resistant (eg, with obesity, receiving supraphysiologic glucocorticoid treatment).
4. Insulin regimens: It is important to note that there is no uniformly accepted “best available” way of prescribing insulin and monitoring its effects. In general, in type 2 DM all insulin regimens should be combined with metformin, if not contraindicated. Insulin therapy should not be unduly delayed if the patient has significant hyperglycemia, because persistent hyperglycemia and elevated proinsulin levels accelerate the progression of DM complications.
1) Single-dose insulin program: Typically used in patients with type 2 DM in whom combined treatment with 2 or 3 oral antidiabetic agents (with or without a GLP-1 receptor agonist) is ineffective and who are transitioning to insulin therapy. One injection of intermediate-acting insulin (NPH) or a long-acting insulin analogue (eg, glargine, detemir, or degludec) is given once a day at about the same time. Patients with high FPG levels are commonly advised to administer insulin at bedtime, while patients with normal FPG levels and daytime hyperglycemia are advised to administer insulin in the morning before breakfast. Preprandial glucose targets are individualized (eg, glucose levels between 4.4 and 6.7 mmol/L [80-130 mg/dL] in younger patients without major comorbidities or between 5.6 and 7.8 mmol/L [100-140 mg/dL] in elderly patients with long-standing DM). At least 4 hours should elapse between a meal and subsequent preprandial measurement. In patients with persistently elevated HbA1c levels despite a single-dose insulin program or doses >0.5 IU/kg/d, a more complex insulin regimen is frequently needed. Once prandial insulin is added, oral insulin secretagogues should be discontinued due to less benefit and in order to decrease the risk of hypoglycemia.
Patients using a single dose of insulin are instructed to monitor their capillary glucose levels before breakfast and before the evening meal. If blood glucose levels are consistently (eg, for 3 consecutive days) above the individualized target range before breakfast and before the evening meal, the insulin dose should be increased by 10% to 20%. If blood glucose levels are consistently below the individualized target range before breakfast and before the evening meal, the insulin dose should be decreased by 10% to 20%. If unexplained symptomatic hypoglycemia occurs at any time (despite consuming adequate meals), the insulin dose may be too high and therefore should be decreased by 10% to 20%. If blood glucose levels are consistently within the individualized target range at one time of the day but consistently outside the individualized target range at another, the single-dose insulin program likely needs to be changed.
2) Split-dose intermediate-acting insulin program: A split-dose intermediate-acting insulin program with NPH insulin twice a day is used by some patients with type 2 DM. Patients following this program take one NPH insulin injection 30 minutes before breakfast and one NPH insulin injection 30 minutes before the evening meal or at bedtime (depending on the patient’s sleep habits, early morning hyperglycemia between 2:00 and 8:00—the “dawn phenomenon”—may be better controlled with NPH insulin given at bedtime because of the timing of its peak effect). Capillary blood glucose measurements before breakfast and before the evening meal are required to estimate if the insulin doses are appropriate. For the morning dose adjustments, blood glucose measurements before the evening meal are evaluated. For the evening dose adjustments, blood glucose measurements before breakfast of the following day are evaluated. The insulin doses can be adjusted by 10% to 20% based on personalized preprandial glycemic targets. Patients following this program need a diet that has a consistent amount of carbohydrates and have to eat their meals at about the same time every day.
3) Split mixed-dose insulin program: A split mixed-dose insulin program with intermediate-acting insulin (NPH) plus either short-acting human insulin (regular) or rapid-acting insulin analogue (aspart, lispro, or glulisine) administered each with breakfast and the evening meal is occasionally used. Before breakfast, patients on this program take an injection of NPH insulin plus an injection of either rapid-acting insulin or short-acting insulin. Before the evening meal, they also get an injection of NPH insulin plus an injection of one of the prandial insulin preparations. Patients are instructed to check their capillary glucose levels before breakfast, before the noon meal, before the evening meal, and at bedtime. They need to follow a diet that has a consistent amount of carbohydrates and eat their main meals at about the same time every day. Glucose measurements before breakfast indicate the effectiveness of the evening-meal NPH insulin administered the previous day. Glucose measurements before the noon meal indicate the effectiveness of the breakfast rapid-acting insulin (or short-acting insulin). Glucose measurements before the evening meal indicate the effectiveness of the breakfast NPH insulin dose. Glucose measurements before bedtime indicate the effectiveness of the evening-meal prandial insulin. The insulin doses are changed by 10% to 20% during each dose adjustment.
4) Premixed split-dose insulin program: In a premixed split-dose insulin program one of the premixed insulin preparations (Table 6.2-4) is administered twice a day, before breakfast and before the evening meal. A common practice is to initially give ~60% of the total daily insulin doses in the morning and ~40% in the evening. Patients are instructed to check their capillary blood glucose levels before breakfast, before the noon meal, before the evening meal, and at bedtime. Patients need to follow a diet that has a consistent amount of carbohydrates and eat their main meals at about the same time every day. Hypoglycemia could be the consequence, for example, of skipping or delaying a meal, eating fewer carbohydrates than usual, or doing an unusual amount of physical activity. In this program glucose measurements before the noon meal and before the evening meal indicate the effectiveness of the morning premixed insulin dose. Glucose measurements before bedtime and before breakfast the next day indicate the effectiveness of the evening premixed insulin dose.
If blood glucose values before the noon meal and before the evening meal are both consistently (eg, for 3 consecutive days) above or below the individualized target range, the breakfast premixed insulin dose should be increased or decreased by 10% to 20%. If blood glucose values at bedtime and before breakfast the next day are both consistently above or below the individualized target range, the evening-meal premixed insulin dose should be increased or decreased by 10% to 20%.
5) Multiple daily injection insulin program: This is the principal method of treatment of type 1 DM that is also recommended in patients with type 2 DM who require full insulin replacement and insulin injections 4 times a day. Typically the program consists of a combination of long-acting basal insulin (eg, glargine, detemir, or degludec) given once daily in the morning or evening and rapid-acting insulin (aspart, lispro, or glulisine) with meals 3 times a day.
For current basal insulin users, we maintain the basal dose and add bolus insulin with each meal at a dose equivalent to 10% of the basal dose.
For new insulin users, we consider starting a full basal dose in addition to the bolus regimen: we calculate the total daily insulin (TDI) dose as 0.3 to 0.6 IU/kg/d and then distribute this into basal insulin at bedtime or morning (40% of the TDI dose) and prandial (bolus) insulin prior to each meal (20% of the TDI dose).
Titration depends on capillary glucose measurements and HbA1c. This basal-bolus regimen is supplemented by correction scales that add units to or subtract them from the rapid-acting insulin prandial doses. Patients are instructed to check their capillary blood glucose levels before breakfast, before the noon meal, before the evening meal, and at bedtime. Basal insulin requirements are based on overnight glucose readings, as this period is not affected by the effects of mealtime insulin or different activities. When the dose of a long-acting insulin analogue is appropriate, overnight blood glucose levels (difference between glucose readings at bedtime and before breakfast) should remain stable (eg, not increasing or decreasing by >2.2 mmol/L [40 mg/dL]). If blood glucose levels consistently drop overnight (eg, >2.2 mmol/L [40 mg/dL] between bedtime and breakfast), the dose of the long-acting insulin should be decreased by 10% to 20%. If blood glucose levels consistently rise overnight (eg, >2.2 mmol/L [40 mg/dL] between bedtime and breakfast), the dose of the long-acting insulin should be increased by 10% to 20%.
To adjust the prandial insulin doses, the blood glucose values before the next meal (or at bedtime) should be assessed. Glucose measurements before the noon meal indicate the effectiveness of the breakfast rapid-acting insulin. Glucose measurements before the evening meal indicate the effectiveness of the noon-meal rapid-acting insulin. Glucose measurements before bedtime indicate the effectiveness of the evening-meal rapid-acting insulin. The objective is to keep the preprandial glucose values (typically 4.4-7 mmol/L [80-130 mg/dL]) and bedtime glucose values within the individualized glycemic target. Another way to adjust prandial insulin doses is to check postprandial glucose levels (1-2 hours after the beginning of a meal) targeting levels <10 mmol/L (180 mg/dL) and 5 to 8 mmol/L if safely achievable. Prandial insulin doses depend on how many carbohydrates are present in diet. Two strategies can be followed when planning the prandial insulin therapy:
a) Fixed meal insulin doses following a diet consistent in carbohydrates on consecutive days.
b) Carbohydrate counting: For the purpose of carbohydrate counting, the ratio of insulin to grams of carbohydrates (number of grams of carbohydrates covered by 1 IU of rapid-acting insulin) is calculated for each meal. Usually the ratio is started with 1 IU of insulin for every 15 g of carbohydrates and the scale is readjusted individually.
6) Continuous subcutaneous insulin infusion (CSII) or insulin pump therapy: Insulin pumps are devices designed to administer short-acting or rapid-acting insulin analogues in a subcutaneous infusion, providing both a continuous basal infusion (40%-60% of total daily insulin dose) and mealtime boluses. Insulin pumps allow for programming delivery for multiple basal rates. The dose of prandial boluses is based on the estimated meal carbohydrate content and capillary blood glucose level immediately before each meal. Insulin pumps also have features that allow for adjustments for the “residual insulin” action from previous boluses, potentially reducing the risk of hypoglycemia from frequent administration of boluses.
The advantages of insulin pump therapy include fewer injections, possibility of giving very low doses of insulin (doses as low as 0.05 IU can be accurately delivered, a feature particularly useful in small children), possibility of delivering >1 basal rate (useful, eg, for treating the dawn phenomenon or for patients with different basal requirements during periods of intense physical activity), and lifestyle flexibility with respect to eating schedules. There is also evidence indicating that in motivated patients properly trained on pump management skills, CSII can provide better glycemic control and lower risk of severe hypoglycemia. Insulin pump therapy is not recommended for patients who are unwilling or unable to perform a minimum of 4 blood glucose tests per day. CSII requires patient training in the fundamental aspects of intensive insulin therapy, carbohydrate counting, and manipulation of insulin pump settings. Potential risks associated with insulin pump therapy include blockage or leakage of the system (leading to rapid hyperglycemia and potentially DKA in patients with type 1 DM), infections at the site of infusion, and hypoglycemia (eg, if the basal insulin dose is too high and the patient skips a meal). Another disadvantage is the high cost of the pump and supplies.
rtCGM systems measure the interstitial fluid glucose level, which is generally within 15% to 20% of the capillary glucose concentration, and provide semicontinuous real-time information about glucose levels that identifies fluctuations difficult to assess with conventional capillary blood glucose self-monitoring. These systems are now commonly used in conjunction with CSII (a sensor-augmented insulin pump) to provide more detailed information about the patients’ glycemic patterns (eg, average glucose, percentage of hypoglycemic and target ranges). CGM systems can play a valuable role in the management of patients with hypoglycemia unawareness and hyperglycemic excursions and are highly recommended in children and adolescents with type 1 DM. There are also other devices that allow for intermittent measuring of glucose levels, but the measurements are only obtained on demand. Some sensor-augmented pumps can be programmed to interrupt insulin delivery for up to 2 hours at a preset sensor glucose value (threshold-suspend feature). This feature can reduce the frequency of nocturnal hypoglycemia and severe hypoglycemia without increasing HbA1c values or causing DKA. Newer CGM devices do not require capillary blood glucose measurements to calibrate the sensors.
Overall, CSII (particularly sensor-augmented insulin pumps with the threshold-suspend feature) should be offered to individuals with type 1 DM in whom there is concern about hypoglycemia unawareness or high risk of severe hypoglycemia,Evidence 13Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). Moderate Quality of Evidence (moderate confidence that we know true effects of intervention). Quality of Evidence lowered as some critical patient-important outcome measures have not been explored. Misso ML, Egberts KJ, Page M, O'Connor D, Shaw J. Continuous subcutaneous insulin infusion (CSII) versus multiple insulin injections for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2010 Jan 20;(1):CD005103. doi: 10.1002/14651858.CD005103.pub2. Review. PMID: 20091571. Langendam M, Luijf YM, Hooft L, Devries JH, Mudde AH, Scholten RJ. Continuous glucose monitoring systems for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2012 Jan 18;1:CD008101. doi: 10.1002/14651858.CD008101.pub2. Review. PMID: 22258980. patients requiring very low doses of insulin that cannot be given by syringes or pens, or possibly those who wish a tighter glycemic control with more flexible eating schedules.Evidence 14Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). Low Quality of Evidence (low confidence that we know true effects of intervention). Quality of Evidence lowered as some critical patient-important outcome measures have not been explored. Misso ML, Egberts KJ, Page M, O'Connor D, Shaw J. Continuous subcutaneous insulin infusion (CSII) versus multiple insulin injections for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2010 Jan 20;(1):CD005103. doi: 10.1002/14651858.CD005103.pub2. Review. PMID: 20091571. Langendam M, Luijf YM, Hooft L, Devries JH, Mudde AH, Scholten RJ. Continuous glucose monitoring systems for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2012 Jan 18;1:CD008101. doi: 10.1002/14651858.CD008101.pub2. Review. PMID: 22258980. All such patients should be willing and able to learn the complexities of CSII therapy and follow closely their glycemic patterns.
Pharmacotherapy: Noninsulin Therapies
1. When choosing an antidiabetic medication for patients with type 2 DM, the glucose-lowering efficacy, safety profile, tolerability, convenience, patient preferences, comorbidities, concurrently used drugs, adverse effects, pregnancy, contraindications, and costs of available agents should be considered. The effect on weight and the risk of causing hypoglycemia are also important to review. Demonstrated reductions in mortality, CVD, HF, progression of kidney disease, as well as cardiovascular and renal risk are additional major factors that should be considered in the initial selection of treatment.
2. A patient-centered personalized approach with shared decision-making is recommended. Although there are uncertainties regarding the best choice and sequence of therapy, the general consensus is that metformin is used as the initial drug for treatment of type 2 DM if there are no contraindications (eg, advanced renal failure). Metformin has a relatively strong glucose-lowering effect, possible cardiovascular benefits, and proven long-term safety, and it is widely available at a low cost. However, there is increasing evidence that GLP-1 analogues and SGLT-2 inhibitors provide a variety of cardiovascular and renal benefits regardless of HbA1c in high-risk patients. According to the 2022 ADA and European Association for the Study of Diabetes (EASD) practice guidelines, in patients with established ASCVD (eg, history of MI, stroke, any revascularization procedure) or those aged ≥55 years with ≥2 cardiovascular risk factors (including obesity, hypertension, smoking, dyslipidemia, or albuminuria), GLP-1 receptor agonists (liraglutide, semaglutide, and dulaglutide) or SGLT-2 inhibitors (canagliflozin, empagliflozin, dapagliflozin) should be used to reduce major adverse cardiovascular events (MACEs) and may be combined if HbA1c is above the target level. In patients with current or prior symptoms of HF, SGLT-2 inhibitors with proven HF benefit should be used to reduce hospitalization for HF. In patients with CKD (estimated glomerular filtration rate [eGFR]<60 mL/min/1.73m2 with or without albumin-creatinine ratio [ACR] ≥30 mg/mmol), SGLT-2 inhibitors with primary evidence of reducing CKD progression should be preferably used to reduce the progression of nephropathy. SGLT-2 inhibitors can be initiated in people with an eGFR ≥20 mL/min/1.73m2 and continued until dialysis or transplant. GLP-1 receptor analogues can be used as an alternative of SGLT-2 inhibitors in case of intolerance or contraindications, or they may be combined if HbA1c is above the target level (Figure 6.2-4 and Figure 6.2-5). In patients with established or high risk of CVD, heart failure, and/or CKD, the use of metformin should not delay the start of those agents but could certainly be an adjunct to improve glycemic control.
3. In patients with type 2 DM progression or those in whom the first medication (usually metformin) is contraindicated or has failed to meet the individualized glycemic targets, a stepwise therapy with the addition of other oral or injectable medications (including insulin) is frequently needed. Treatment should be individualized on a case-by-case basis rather than based on rigid application of a single possible algorithm.
The therapeutic goal is to achieve and maintain glucose targets (HbA1c and/or TIR) and avoid both hypoglycemia and treatment inertia. Of note, the 2022 ADA/EASD consensus report groups medications according to their efficacy in glucose lowering: (1) very high: dulaglutide (high dose), semaglutide, tirzepatide, insulin, combination oral agents, combination injectable agents (GLP-1 receptor agonists/insulin); (2) high: other GLP-1 receptor agonists not listed above, metformin, SGLT-2 inhibitors, sulfonylurea, TZDs; (3) intermediate: DPP-4 inhibitors.
The benefits in terms of HbA1c reduction, cardiorenal outcomes, convenience, and weight effects, as well as the downsides of each medication (regarding age and frailty, hypoglycemia risk, contraindications, cost, pregnancy) need to be personalized, and patient preferences should also be taken into account. Dosage, mechanism of action, advantages, and disadvantages of available antidiabetic agents: Table 6.2-5.
4. The ADA/EASD 2022 consensus report suggests that weight loss of 5% to 15% could be a primary management target for many patients with type 2 DM, but weight management goals should be individualized. Greater weight loss generally results in better outcomes and may exert benefits beyond glycemic management, improving other risk factors for cardiometabolic diseases and quality of life.
Of note, this consensus report groups diabetic medications according to their efficacy for weight loss: (1) very high: semaglutide, tirzepatide; (2) high: dulaglutide, liraglutide; (3) intermediate: other GLP-1 receptor agonists (not listed above), SGLT-2 inhibitors; (4) neutral: DPP-4 inhibitors, metformin. When choosing glucose-lowering therapy in patients with obesity, its effect on weight should also be considered, with a preference for regimens with high to very high dual efficacy on glucose and weight.
5. Shared decision-making tools have been developed and can be used as aids that enable conversations resulting in DM treatment regimens consistent with patients’ preferences and values.
6. Do not delay insulin therapy in patients in whom it is indicated, especially in the setting of metabolic decompensation or significant symptoms of hyperglycemia.
7. Always adjust doses of oral antidiabetic agents to achieve glycemic targets and based on renal function. Dose adjustment is also recommended to avoid hypoglycemia when adding a new agent to a regimen containing insulin, sulfonylurea, or glinide therapy, particularly in patients at or near glycemic goals and in CKD (see Follow-Up, below).
Hypoglycemia is defined by blood glucose levels <3.9 mmol/L (70 mg/dL). Its severity is further classified as level 1 (≥3.0 mmol/L [54 mg/dL]), level 2 (<3.0 mmol/L [54 mg/dL]; sufficiently low to indicate serious, clinically important hypoglycemia), and level 3 (altered mental and/or physical status requiring assistance of a third party for recovery; see Drug-Induced Hypoglycemia). Patients with DM should learn to recognize the symptoms of hypoglycemia (eg, sweating, tremors, weakness, hunger) and learn how to treat it. The “rule of 15” can be used to treat conscious patients with level 1 or level 2 hypoglycemia:
1) Check the capillary glucose level immediately when there are symptoms of hypoglycemia.
2) If the patient is awake and able to swallow, administer 15 g of carbohydrates (eg, 4 glucose tablets [4 g each], 100-125 mL of fruit juice, 1 tablespoon of sugar or syrup).
3) Wait 15 minutes and recheck the blood glucose level.
4) If the capillary blood glucose level remains low or if there are still symptoms of hypoglycemia, treat the patient again with 15 g of carbohydrates and continue to recheck the capillary glucose levels until the target glucose levels are reached and symptoms resolve.
In patients with level 3 hypoglycemia and those who are unwilling or unable to swallow, treatment with IV glucose or nasal or IM glucagon injection (or both) should be provided immediately.
Patients with DM receiving insulin therapy with a history of level 2 hypoglycemia should have a glucagon injection or glucagon nasal spray available (see Drug-Induced Hypoglycemia).
Serious Intercurrent Illness and Sick-Day Guidelines
Acute illnesses frequently lead to worsening of hyperglycemia and increased insulin requirements. Patients with type 1 or type 2 DM on insulin or insulin secretagogues likely benefit from learning management strategies that can be applied during acute sickness (“sick-day guidelines”). These strategies include monitoring blood glucose levels more frequently, keeping good hydration, avoiding exercise as it may worsen hyperglycemia, checking for urine ketones if there is severe and persistent hyperglycemia, using extra doses of rapid-acting insulin to temporarily correct hyperglycemia—“insulin supplements”—and knowledge of when to contact health-care providers. For patients treated with oral agents for type 2 DM, education should be provided on withholding SADMANS medications (sulfonylurea, ACEIs, diuretics, metformin, ARBs, nonsteroidal anti-inflammatory drugs [NSAIDs], and SGLT-2 inhibitors) when they are ill and unable to maintain adequate hydration to reduce the risk of kidney dysfunction and, particularly, euglycemic DKA with SGLT-2 inhibitors.
1. Whole pancreas transplant is most frequently used in patients with renal failure in whom pancreas transplant is combined with kidney transplant.
2. Pancreatic islet transplant is associated with lower risk than whole pancreas transplant and allows for the normalization of blood glucose levels. Its use is limited by poor graft survival.
Follow-UpTop
1. Glycemic control: The ADA and Diabetes Canada recommend checking HbA1c levels based on clinical situation. For patients with well-controlled DM, testing twice per year is appropriate. For unstable or highly intensively managed patients, testing every 3 months is appropriate.
2. Screening for hypertension: The ADA advises measuring blood pressure at or before every routine medical visit, preferably with automated office blood pressure monitoring to reduce the white coat effect (see Essential Hypertension). Elevated values should be confirmed on a separate day and preferentially by home or 24-hour ambulatory blood pressure monitoring.
3. Screening for dyslipidemia: The Canadian Cardiovascular Society and Diabetes Canada recommend measuring the lipid profile at least annually in most adult patients with DM.
4. Screening for chronic complications of DM:
1) Nephropathy: The ADA advises performing an annual test to quantitate urine albumin excretion (ACR) in patients with type 1 DM lasting ≥5 years and in all patients with type 2 DM since the time of diagnosis. Serum creatinine with eGFR should also be measured at least annually.
2) Retinopathy: The ADA recommends that patients with type 1 DM should have an initial dilated and comprehensive eye examination by an ophthalmologist or optometrist within 5 years after the onset of DM. In patients with type 2 DM this should be done shortly after the diagnosis of DM. If there is no evidence of retinopathy for ≥1 eye examination, then examinations every 2 years may be considered. If diabetic retinopathy is present, subsequent examinations should be repeated at least annually or more frequently as per ophthalmologic recommendations.
3) Diabetic foot complications: The International Working Group on Diabetic Foot Editorial Board and the ADA recommend that all patients with DM should have a thorough foot examination at least once a year, and more frequently if high-risk foot conditions (eg, diabetic peripheral neuropathy, foot deformities, or peripheral artery disease) are identified. The ADA also advises that visual inspection of the feet should be performed at every health-care visit.
4) Other complications: The ADA recommends screening patients regularly for symptoms of diabetic autonomic dysfunction. These include orthostatic hypotension, resting tachycardia, esophageal motility disorders, gastroparesis, constipation, diarrhea, fecal incontinence, as well as erectile dysfunction.
PreventionTop
1. Type 1 DM: Until recently, there were no effective methods of prevention, but the use of novel immunotherapies looks promising in delaying the occurrence of type 1 DM in high-risk patients.Evidence 15Moderate Quality of Evidence (moderate confidence that we know true effects of intervention). Quality of Evidence lowered due to indirectness and imprecision. Herold KC, Bundy BN, Long SA, et al; Type 1 Diabetes TrialNet Study Group. An Anti-CD3 Antibody, Teplizumab, in Relatives at Risk for Type 1 Diabetes. N Engl J Med. 2019 Aug 15;381(7):603-613. doi: 10.1056/NEJMoa1902226. Epub 2019 Jun 9. Erratum in: N Engl J Med. 2020 Feb 6;382(6):586. PMID: 31180194; PMCID: PMC6776880.
2. Type 2 DM: Intensive and structured healthy behavior interventions, ideally resulting in loss of ~5% of initial body weight, can reduce the risk of progression from IFG or IGT to type 2 DM by ~60%. Progression from prediabetes to type 2 DM can also be reduced by pharmacologic therapy with metformin (~30% reduction), with persistent benefits observed after >10 years of stopping treatment in the Diabetes Prevention Program. TZDs showed beneficial effects on delaying the development of type 2 DM (at the expense of using diabetic drugs), but the multiple potential adverse effects and warnings in this class of medication make it difficult to recommend their use in people with IFG or IGT. Acarbose has also been used to reduce the risk of progression to type 2 DM, but its effect did not persist after discontinuation. Liraglutide has been shown to prevent IGT conversion to type 2 DM and cause reversion to normoglycemia, but some limitations to recommend its use to prevent type 2 DM include the fact that individuals were not followed up after discontinuation, the cost effectiveness of the active therapy compared with healthy behavior interventions alone, and questionable long-term adverse effects. Finally, even though bariatric surgery performed in people with obesity (BMI >30 kg/m2) at risk for type 2 DM showed a major reduction in progression to type 2 DM, evidence from randomized clinical trials and cost-benefit analysis of bariatric surgery as a primary tool to prevent DM are still missing. Hence, more data are needed before recommending bariatric surgery to prevent DM.
Tables and FiguresTop
|
Differential features |
LADA |
Type 2 diabetes mellitus |
|
Body mass index |
As in general population |
Obesity or overweight |
|
Hypertension |
No |
Yes |
|
Family history of diabetes |
No |
Yes |
|
History/family history of autoimmune diseases |
Yes |
No |
|
Anti-GAD or other islet cell antibodies |
Yes |
No |
|
C-peptide (glucagon test) |
Low level |
Normal or initially increased |
|
Treatment of choice |
Insulin |
Initial treatment with oral antidiabetic agents |
|
GAD, glutamic acid decarboxylase; LADA, latent autoimmune diabetes in adults. | ||
|
Differential features |
MODY |
Type 1 diabetes mellitus |
|
Congenital malformations (most frequently affecting the kidneys and urogenital system) |
Possible |
No |
|
≥3 generations of family history of diabetes mellitus at age <30 years |
Yes |
No |
|
History/family history of autoimmune diseases |
No |
Yes |
|
Islet cell antibodies |
No |
Yes |
|
C-peptide level |
Initially normal |
Low |
|
Treatment of choice |
Initial treatment with sulfonylureas |
Insulin |
|
Onset |
Slow and often asymptomatic |
Usually acute |
|
MODY, maturity-onset diabetes of youth. | ||
|
Insulin preparations |
Time of action | |||
|
Onset |
Peak |
Effective duration | ||
|
Rapid-acting insulin analogues |
Aspart |
5-20 min |
40-75 min |
3-5 h |
|
Glulisine |
5-20 min |
40-75 min |
3-5 h | |
|
Lispro |
5-20 min |
40-75 min |
3-5 h | |
|
Short-acting insulins |
Regular |
30 min |
2-4 h |
5-8 h |
|
Intermediate-acting insulins |
NPH (isophane) |
2 h |
4-12 h |
10-18 h |
|
Long-acting insulin analogues |
Detemir |
2 h |
3-9 h |
8-24 h |
|
Glargine (100 IU/mL; also available as 300 IU/mL) |
2 h |
No peak |
20-36 h | |
|
Degludec (100 IU/mL; also available as 200 IU/mL) |
2 h |
No peak |
42 h | |
|
NPH, neutral protamine Hagedorn. | ||||
|
Types of insulins in the combination |
Brand names |
|
Premixed human insulin |
Humulin® 20/80; Humulin® 70/30; Novolin® ge 30/70; Novolin® ge 40/60; Novolin® ge 50/50 |
|
Aspart plus aspart protamine suspension |
NovoMix® 30 |
|
Lispro plus lispro protamine suspension |
Humalog® Mix 25 |
|
Biguanides | ||
|
Drug and dosage |
Metformin: Initially 500 or 850 mg PO once daily taken with largest meal. Dose increased by 500 mg/wk up to usual dose of 1000 mg bid (with meals). Max dose 2 g/d | |
|
Commentsa |
Mechanism of action: ↓ liver production of glucose Efficacyb: HbA1c ↓ 1%-2% Contraindications: Renal failure with GFR <30 mL/min/1.73 m2 (or serum creatinine ≥132.6 micromol/L [1.5 mg/dL] in men or ≥123.8 micromol/L [1.4 mg/dL] in women according to manufacturer), severe hepatic dysfunction, decompensated or advanced HF, acidosis, hypoxia, shock, history of severe hypersensitivity to metformin Frequent adverse effects: Diarrhea, nausea, vomiting, bloating, abdominal cramping, metallic taste Rare adverse effects: Lactic acidosis Risk of hypoglycemia: No if monotherapy Effect on weight: Neutral or modest weight loss Miscellaneous advantages: Extensive experience, low cost Miscellaneous disadvantages: Can cause vitamin B12 deficiency. Manufacturer recommends temporarily discontinuing metformin in patients undergoing radiologic studies where intravascular iodinated contrast media are used Other comments: GI adverse effects more frequent early in the course of treatment. Extended-release metformin may be better tolerated in patients with GI adverse effects. Elderly patients should not be titrated to max dose. Careful use in patients ≥80 years (normal renal function has to be established) | |
|
Sulfonylureas | ||
|
Drug and dosage |
Glipizide: 2.5-20 mg PO once daily. Doses >15 mg/d should be administered in 2 divided doses. Immediate-release tablets should be administered 30 min before meals (typically before breakfast if once daily); extended-release tablets should be given with breakfast. Titrate in 2.5-5 mg increments. Max recommended dose 20 mg/d Glimepiride: 1-8 mg PO once daily. Administer once daily with breakfast or first main meal of the day. Titrate in 1-2 mg increments. Max recommended dose 8 mg/d Glyburide: 1.25-20 mg PO once daily. Patients receiving >10 mg daily may have more satisfactory response with bid dosing. Administer with meals (typically before breakfast or first main meal of the day if once daily). Titrate in 1.25-2.5 mg increments. Max recommended dose 20 mg/d Micronized glyburide (greater bioavailability): 0.75-12 mg PO once daily. Patients receiving >6 mg daily may have more satisfactory response with bid dosing. Administer with meals (typically before breakfast or first main meal of the day if once daily). Titrate in 0.75-1.5 mg increments. Max recommended dose 12 mg/d Gliclazide: 80-320 mg/d in 2 divided doses, 30 min before meals. Modified-release tablets 30 mg once daily (with breakfast). Increase dose (by 30 mg every 2 weeks) up to max of 120 mg/d | |
|
Commentsa |
Mechanism of action: Closes KATP channels on beta-cell plasma membranes. ↑ insulin secretion Efficacyb: HbA1c ↓ 1%-2% Contraindications: History of severe hypersensitivity reactions Frequent adverse effects: Hypoglycemia, lack of energy and strength Rare adverse effects: Diarrhea, nausea, constipation, flatulence, dizziness, headaches Risk of hypoglycemia: Present, particularly in the elderly, with strenuous exercise, or due to interactions with other drugs (eg, sulfonamides, alcohol) Effect on weight: Modest gain Miscellaneous advantages: Extensive experience, low cost Miscellaneous disadvantages: Failure rate may exceed other drugs (this is attributed to exacerbation of islet dysfunction) Other comments: Reduces postprandial glucose excursions. Usually start with lowest dose and increase every 1-2 weeks based on blood glucose. Patients with decreased caloric intake or fasting may need doses held to avoid hypoglycemia. Long-acting sulfonylureas (eg, glyburide) may be associated with higher risk of hypoglycemia than short-acting sulfonylureas (eg, glipizide, glimepiride) | |
|
Meglitinides (glinides) | ||
|
Drug and dosage |
Repaglinide: 0.5-4 mg PO 1-30 min before each meal 2, 3, or 4 times/d based on meal pattern. Titrate in 1-2 mg increments weekly. Max recommended dose 16 mg/d Nateglinide: 60-120 mg PO 1-30 min before each meal 2, 3, or 4 times/d based on meal pattern | |
|
Commentsa |
Mechanism of action: Closes KATP channels on beta-cell plasma membranes. ↑ insulin secretion Efficacyb: HbA1c ↓ 1%-2% Contraindications: History of severe hypersensitivity reactions Frequent adverse effects: Headaches Rare adverse effects: Diarrhea, arthralgias Risk of hypoglycemia: Present (possibly smaller than with sulfonylureas) Effect on weight: Modest gain Miscellaneous advantages: Both can be used in patients allergic to sulfonylureas. Short duration of action allows for dosing flexibility with meals Miscellaneous disadvantages: Tid dosing, expensive Other comments: Reduces postprandial glucose excursions. Repaglinide is more effective at lowering HbA1c than nateglinide. Repaglinide is principally metabolized by liver with <10% excreted by kidneys (dose adjustments not typically required in patients with renal insufficiency). Nateglinide has active metabolites excreted by kidneys and should be used with caution in renal insufficiency. If patient misses a meal, glinides should not be administered to avoid hypoglycemia | |
|
Alpha-glucosidase inhibitors | ||
|
Drug and dosage |
Acarbose: Initially 25 mg PO tid immediately before main meals (some patients benefit from starting with 25 mg once daily with gradual titration to 25 mg tid to reduce GI adverse effects). Dose may be increased every 2-4 weeks. Max dose 50 mg tid (≤60 kg) or 100 mg tid (>60 kg) | |
|
Commentsa |
Mechanism of action: Inhibits intestinal alpha-glucosidase and slows down the final enzymatic stage of intestinal digestion of polysaccharides, oligosaccharides, and some disaccharides (maltose and sucrose) Efficacyb: HbA1c ↓ 0.5%-1% (mainly ↓ postprandial glucose levels) Contraindications: History of severe hypersensitivity reactions, IBD, colonic ulceration, conditions that may deteriorate due to increased intestinal gas formation, predisposition to intestinal obstruction, partial intestinal obstruction, cirrhosis, renal impairment (serum creatinine >2 mg/dL) Frequent adverse effects: Flatulence, diarrhea, abdominal pain Rare adverse effects: Ileus, hepatotoxicity Risk of hypoglycemia: No if monotherapy Effect on weight: Neutral Miscellaneous advantages: No systemic effects Miscellaneous disadvantages: Frequent GI adverse effects; frequent dosing; expensive Other comments: Reduces postprandial glucose excursions. In case of hypoglycemia (eg, concomitant use of sulfonylureas), glucose (dextrose) recommended for treatment. GI adverse effects may be decreased by restricting dietary sucrose (table sugar) | |
|
Thiazolidinediones (TZDs) | ||
|
Drug and dosage |
Pioglitazone: 15-30 mg PO once daily, administered without regard to meals. Dose can be increased in 15 mg increments with careful monitoring of adverse effects (eg, weight gain, edema, symptoms of HF). Max dose 45 mg once daily Rosiglitazone: 4 mg PO once daily or in divided doses bid, administered without regard to meals. Dose can be increased up to 8 mg daily, as a single daily dose or in divided doses bid. Max dose 8 mg/d | |
|
Commentsa |
Mechanism of action: Activates the nuclear transcription factor PPAR-gamma, ↑ insulin sensitivity Efficacyb: HbA1c ↓ 0.5%-1.5% Contraindications: History of severe hypersensitivity reactions, CHF, serious hepatic impairment, active bladder cancer, history of bladder cancer; uninvestigated macroscopic hematuria, pregnancy Frequent adverse effects: Edema, headaches Rare adverse effects: CHF exacerbation, bone fractures, anemia, possibly bladder cancer (pioglitazone) Risk of hypoglycemia: No if monotherapy Effect on weight: Modest gain Miscellaneous advantages: Effectiveness may be more durable than sulfonylureas and metformin. ↑ HDL-C, ↓ triglycerides Miscellaneous disadvantages: Rosiglitazone may ↑ LDL-C Other comments: Limit max dose of pioglitazone to 15 mg/d—and consider dose reduction of rosiglitazone—when used in combination with strong CYP2C8 inhibitors (eg, gemfibrozil) | |
|
Dipeptidyl peptidase-4 (DPP-4) inhibitors (gliptins) | ||
|
Drug and dosage |
Sitagliptin: 100 mg PO once daily. Administer with or without food. If eGFR 30-50 mL/min/1.73 m2, dose 50 mg once daily; if eGFR is <30 mL/min/1.73 m2, dose 25 mg once daily Linagliptin: 5 mg PO once daily. Administer with or without food. No dosage adjustment necessary for renal impairment Saxagliptin: 2.5-5 mg PO once daily. Administer with or without food. If eGFR is ≤50 mL/min/1.73 m2, dose 2.5 mg once daily. If concomitant use of strong CYP3A4/5 inhibitors, dose 2.5 mg once daily Alogliptin: 25 mg PO once daily. Administer with or without food. If eGFR is 30-60 mL/min/1.73 m2, dose 12.5 mg once daily. If eGFR is <30 mL/min/1.73 m2, dose 6.25 mg once daily | |
|
Commentsa |
Mechanism of action: Inhibition of DPP-4 activity leads to ↑ endogenous incretins (GLP-1 and GIP) after meals, ↑ glucose-dependent insulin secretion Efficacyb: HbA1c ↓ 0.5%-0.8% Contraindications: History of severe hypersensitivity reactions, acute pancreatitis, CHF with saxagliptin treatment (?) Frequent adverse effects: Generally well tolerated Rare adverse effects: Headaches, nausea, possibly pancreatitis Risk of hypoglycemia: No if monotherapy Effect on weight: Neutral Miscellaneous advantages: Can be used in patients with advanced renal disease Miscellaneous disadvantages: Modest glucose-lowering efficacy, expensive Other comments: Limited long-term safety data. Saxagliptin may increase risk of CHF | |
|
Glucagon-like peptide-1 (GLP-1) receptor agonists | ||
|
Drug and dosage |
Exenatide: Immediate release: Initial dose 5 microg SC bid within 60 min prior to 2 main meals (≥6 h apart). After 1 month dose may be increased to 10 microg bid. Extended release: 2 mg once weekly without regard to meals or time of day. Rotate injection sites weekly Liraglutide: Initial dose 0.6 mg SC once daily for 1 week (dose intended to reduce GI symptoms but ineffective for glycemic control), then increase to 1.2 mg once daily. Dose may be increased to 1.8 mg once daily. Administer without regard to meals or time of day. Of note, liraglutide is also indicated at a dose of up to 3 mg SC daily for weight loss in adults with BMI ≥27 kg/m2 and weight-related complications or with obesity (BMI ≥30 kg/m2) Albiglutide: 30 mg SC once weekly. Dose may be increased to 50 mg once weekly. Administer without regard to meals or time of day. Rotate injection sites weekly Dulaglutide: 0.75 mg SC once weekly. Dose may be increased to 1.5 mg once weekly after 1 month. Administer without regard to meals or time of day. Rotate injection sites weekly Lixisenatide: Initial dose 10 microg once daily for 14 days; on day 15 increase to 20 microg once daily. Maintenance dose 20 microg once daily. If dose is missed, administer within 1 h of next meal Semaglutide: Initial SC dose 0.25 mg once weekly for 4 weeks. Increase to 0.5 mg once weekly for ≥4 weeks. If further glycemic control necessary, increase to max 1 mg once weekly. Starting dose of PO semaglutide is 3 mg/d for 30 days. Increase to 7 mg/d with max dose of 14 mg/d. Of note, semaglutide is also indicated at a dose of up to 2.4 mg SC weekly for weight loss in adults with BMI ≥27 kg/m2 and weight-related complications or with obesity (BMI ≥30 kg/m2) | |
|
Commentsa |
Mechanism of action: Stimulation of GLP-1 receptors leads to ↑ glucose-dependent insulin secretion; ↓ glucagon secretion; slow gastric emptying; ↑ satiety Efficacyb: HbA1c ↓ 0.5%-1.5% Proven CV benefit: Liraglutide, semaglutide, and dulaglutide may be considered, as they were shown to reduce the risk of MACE (stroke rather than CV death). No CV benefit demonstrated with exenatide or lixisenatide Reduction of CKD progression: Liraglutide and semaglutide, predominantly with progression of albuminuria Contraindications: History of severe hypersensitivity reactions, history of pancreatitis, severe GI disease (eg, gastroparesis), history or family history of medullary thyroid carcinoma or MEN syndrome type 2; caution with severe renal impairment (exenatide contraindicated if eGFR <30 mL/min/1.73 m2) Frequent adverse effects: Nausea, vomiting, diarrhea, injection site reactions (eg, pruritus, swelling), headaches, dizziness, nervousness Rare adverse effects: Possibly pancreatitis, possibly pancreatic cancer Risk of hypoglycemia: No if monotherapy Effect on weight: Modest to significant weight loss Miscellaneous advantages: Once-weekly preparations can reduce treatment burden Miscellaneous disadvantages: Injectable medications, expensive Other comments: GI adverse effects more frequent early in the course of treatment. Administer SC injections in upper arm, thigh, or abdomen. Some patients develop high titers of antibodies that may ↓ glycemic response. Limited long-term safety data. Oral semaglutide needs to be taken on an empty stomach with a sip of water (≤120 mL) and 30 min prior to any other PO intake | |
|
Dual glucose-dependent insulinotropic polypeptide (GIP)/glucagon-like peptide-1 (GLP-1) receptor agonists | ||
|
Drug and dosage |
Tirzepatide (approved as type 2 DM treatment): Initial SC dose 2.5 mg once weekly for 4 weeks. Increase dosage in 2.5 mg increments once weekly after ≥4 weeks on current dose (max dose 15 mg/wk). Administer once weekly at any time of day, with or without meals | |
|
Commentsa |
Mechanism of action: Selective GIP and GLP-1 receptor agonist that enhances first-phase and second-phase insulin secretion and reduces glucagon levels, both in a glucose-dependent manner; slow gastric emptying; ↑ satiety Efficacy: HbA1c ↓ 1.8%-2.4%; body weight ↓ 5-9 kg Contraindications: Treatment of type 1 DM, appetite suppression or treatment of obesity by other means, personal or family history of medullary thyroid carcinoma or personal history of multiple endocrine neoplasia syndrome type 2, pregnancy, history of pancreatitis Frequent adverse effects: Nausea, diarrhea, vomiting, constipation, dyspepsia, abdominal pain, sinus tachycardia Rare adverse effects: Hypersensitivity reaction, injection site reactions, acute gallbladder disease, pancreatitis Risk of hypoglycemia: No if monotherapy Miscellaneous advantages: Once-weekly preparations can reduce treatment burden Miscellaneous disadvantages: Injectable medications, expensive Other comments: Administer SC injections in the upper arm, thigh, or abdomen. Limited long-term safety data | |
|
Sodium-glucose cotransporter 2 (SGLT-2) inhibitors (flozins) | ||
|
Drug and dosage |
Canagliflozin: 100 mg PO once daily before first meal of day. Dose may be increased to 300 mg once daily. If eGFR is 45-59 mL/min/1.73 m2, dose 100 mg once daily. Also has inhibitory effect on SGLT-1 Dapagliflozin: 5 mg PO once daily. Administer in the morning with or without food. Dose may be increased to 10 mg once daily Empagliflozin: 10 mg PO once daily, may be increased to 25 mg daily Sotagliflozin: Currently an investigational drug, under regulatory review by EMA and FDA for treatment of both type 1 and 2 DM. Also has inhibitory effect on SGLT-1 | |
|
Commentsa |
Mechanism of action: Promote renal excretion of glucose Efficacyb: HbA1c ↓ 0.5%-0.8%. Limited glycemic benefit with eGFR <60 mL/min/1.73 m2 Proven CV benefit: Empagliflozin, canagliflozin, and dapagliflozin; CV benefits exist regardless of eGFR but limited data show benefits with eGFR <25 mL/min/1.73 m2 Reduction of HF: Canagliflozin, dapagliflozin, and empagliflozin Reduction of CKD progression: Canagliflozin, dapagliflozin, and empagliflozin Contraindications: History of severe hypersensitivity reactions, severe renal impairment (GFR <20 mL/min/1.73 m2), severe hepatic impairment, active bladder cancer (only with dapagliflozin), type 1 DM Frequent adverse effects: Vulvovaginal candidiasis, urinary frequency, polyuria Rare adverse effects: Urinary tract infections, symptomatic hypotension (particularly in the elderly), hyperkalemia, euglycemic DKA Risk of hypoglycemia: No if monotherapy Effect on weight: Modest weight loss Miscellaneous advantages: Can decrease blood pressure. Empagliflozin has been shown to reduce mortality among patients with type 2 DM at high risk of CV events. Dapagliflozin and empagliflozin delay progression of CKD and improve CV outcomes in patients with HF, regardless of presence or absence of DM Miscellaneous disadvantages: Uncertain long-term effect of chronic glycosuria, modest glucose-lowering efficacy, expensive, LDL-C levels may increase, careful use in conditions associated with dehydration risk Other comments: Correct volume depletion prior to administration. Limited long-term safety data. May reduce postprandial hyperglycemia by delaying intestinal glucose absorption Warning: Recent FDA review applied to all SGLT-2 inhibitors pointed to risk of euglycemic ketoacidosis and serious urinary tract infections (fewer than 100 cases reported over >1 year) | |
|
a Adapted from the American Diabetes Association and the European Association for the Study of Diabetes 2012 position statement. b Predicted reduction of HbA1c levels with monotherapy (expressed in percentage points). | ||
|
↑, increase; ↓, decrease; bid, 2 times a day; BMI, body mass index; CHF, congestive heart failure; CKD, chronic kidney disease; CV, cardiovascular; DKA, diabetic ketoacidosis; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; EMA, European Medicines Agency; FDA, US Food and Drug Administration; GFR, glomerular filtration rate; GI, gastrointestinal; GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide 1; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein cholesterol; HF, heart failure; IBD, inflammatory bowel disease; LDL-C, low-density lipoprotein cholesterol; MACE, major adverse cardiovascular event; MEN, multiple endocrine neoplasia; PO, oral administration; PPAR-gamma, peroxisome proliferator-activated receptor gamma; SC, subcutaneous; SGLT-2, sodium-glucose cotransporter 2; tid, 3 times a day. | ||
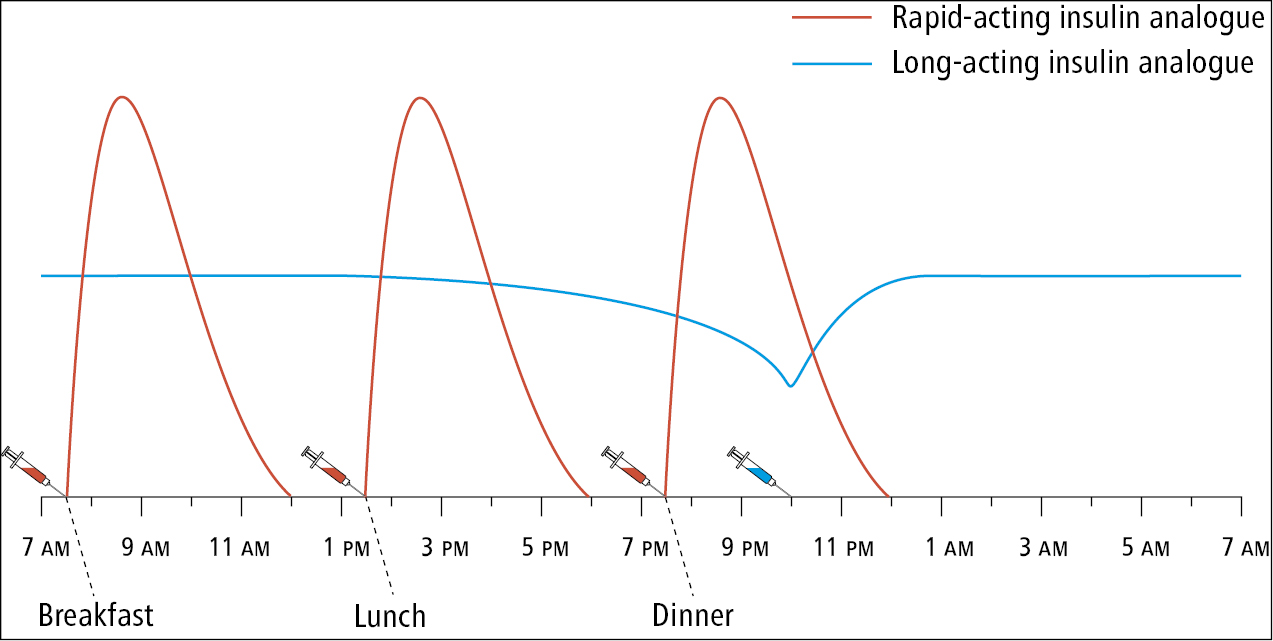
Figure 6.2-1. Intensive insulin therapy regimen with 4 insulin injections a day: a rapid-acting insulin analogue combined with a long-acting insulin analogue.
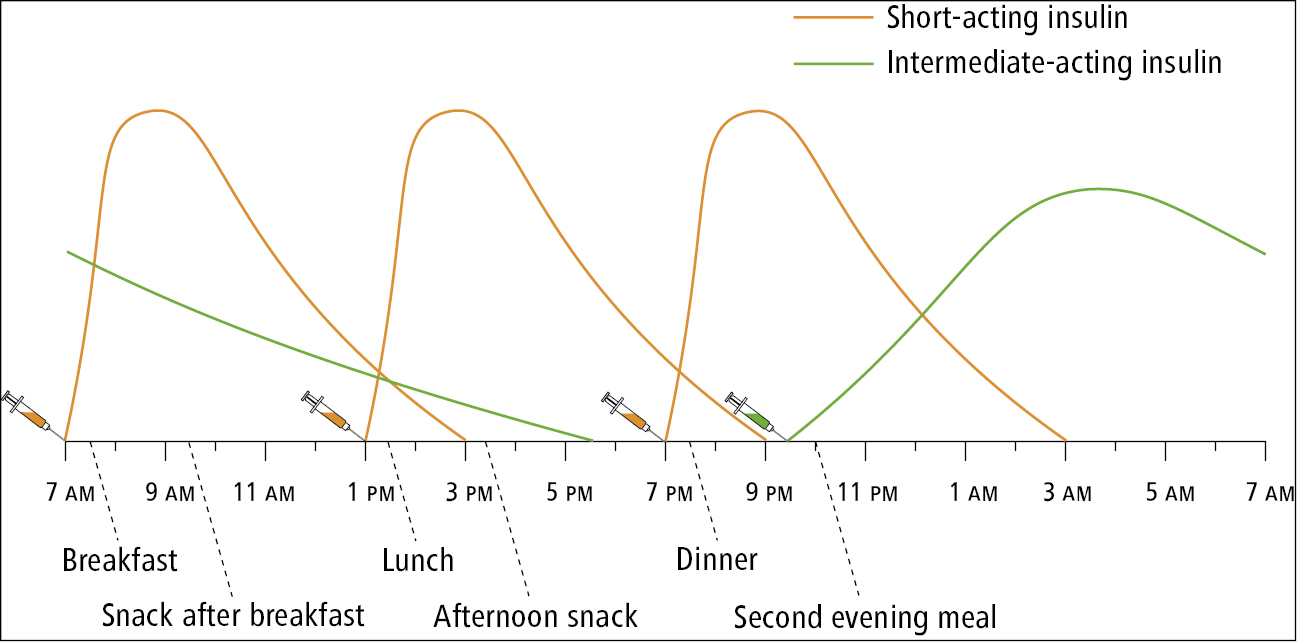
Figure 6.2-2. Intensive insulin therapy regimen with 4 insulin injections a day: a short-acting insulin combined with an intermediate-acting insulin (neutral protamine Hagedorn).
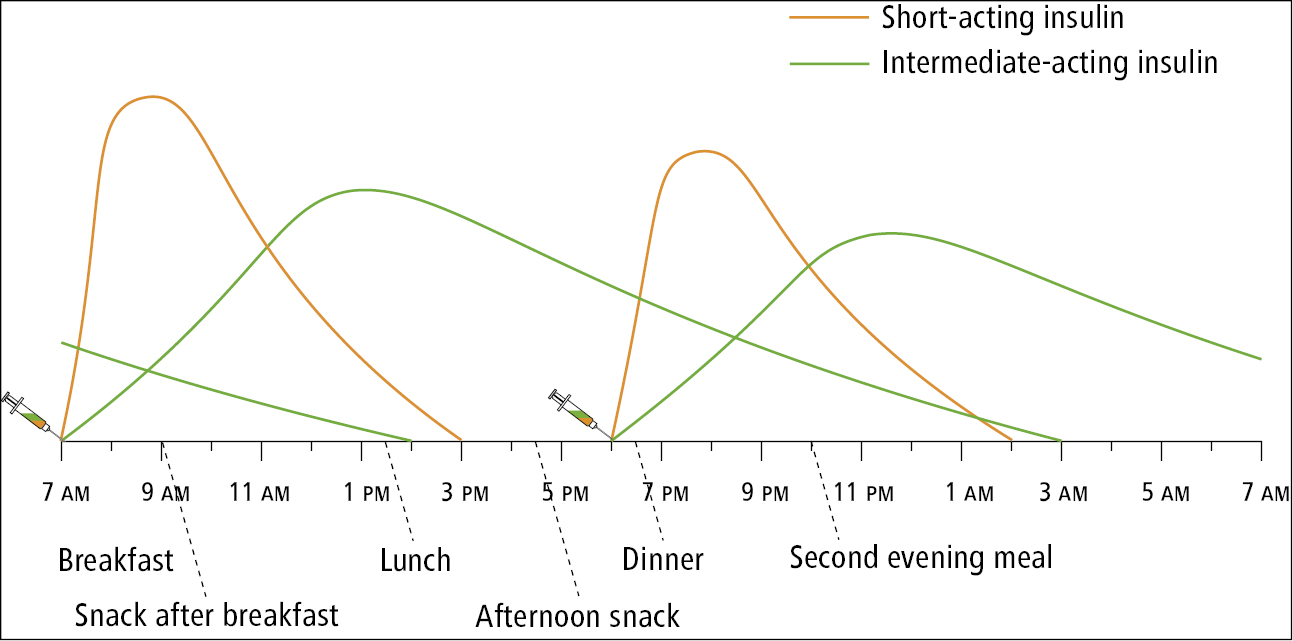
Figure 6.2-3. Treatment regimen with a premixed human insulin (short-acting insulin plus intermediate-acting insulin) administered twice a day.
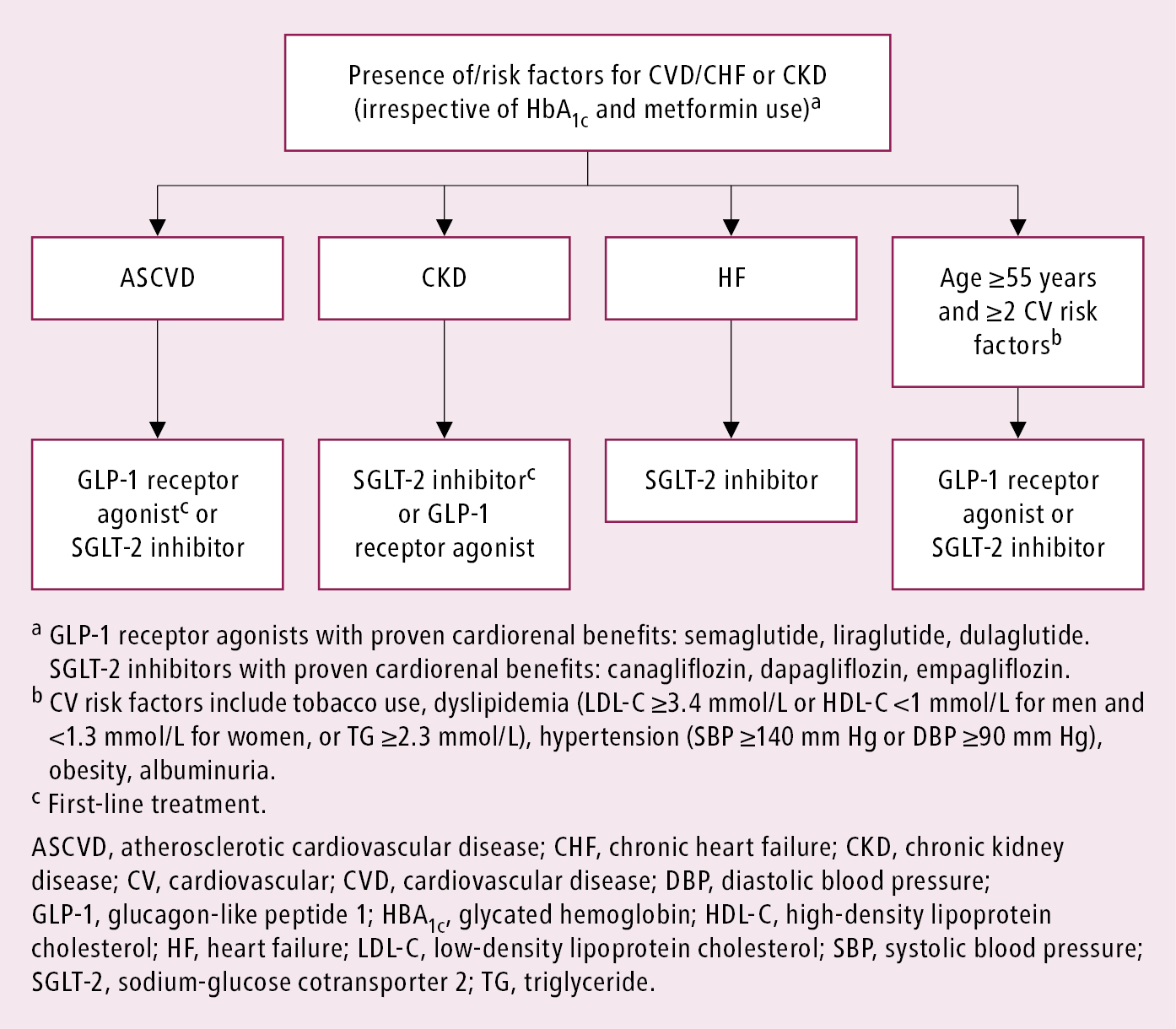
Figure 6.2-4. Algorithm for therapy advancement in type 2 diabetes mellitus based on cardiorenal benefits. Adapted from Can J Diabetes. 2020 Oct;44(7):575-591 and 2022 guidelines from the American Diabetes Association and European Association for the Study of Diabetes.
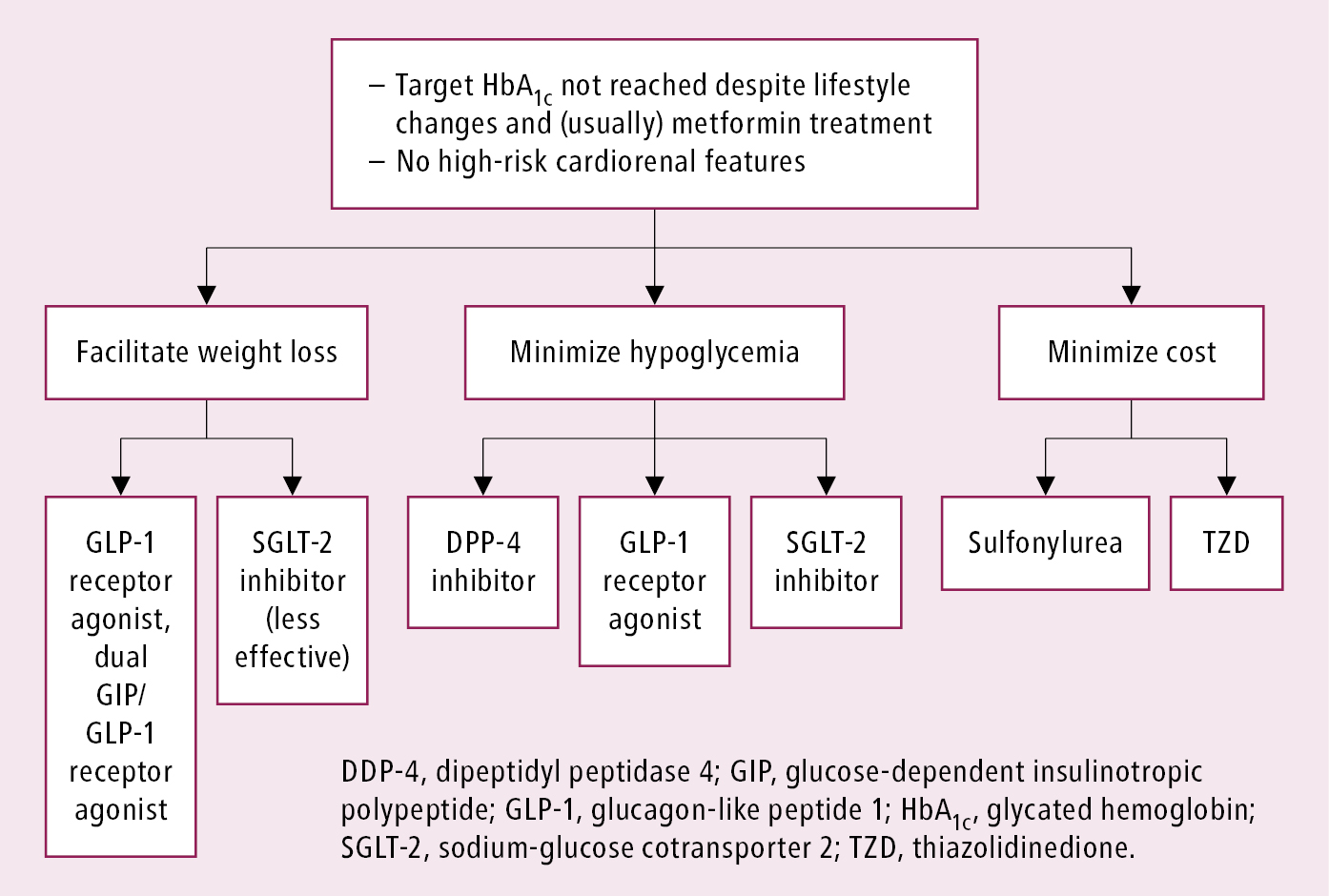
Figure 6.2-5. Algorithm for selection of glucose-lowering agents in patients with diabetes mellitus without high cardiovascular risk. Adapted from Diabetes Care. 2021 Jan;44(Suppl 1):S4-S6 and 2023 American Diabetes Association guidelines.