Herrmann JJ, Brunner-La Rocca HP, Baltussen LEHJM, et al. Liberal fluid intake versus fluid restriction in chronic heart failure: a randomized clinical trial. Nat Med. 2025;31(6):2062-2068. doi:10.1038/s41591-025-03628-4
Solomon SD, McMurray JJV, Vaduganathan M, et al. Finerenone in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N Engl J Med. 2024;391(16):1475-1485. doi:10.1056/NEJMoa2407107
Kosiborod MN, Abildstrøm SZ, Borlaug BA, et al. Semaglutide in Patients with Heart Failure with Preserved Ejection Fraction and Obesity. N Engl J Med. 2023;389(12):1069-1084. doi:10.1056/NEJMoa2306963
Kittleson MM, Panjrath GS, Amancherla K, et al. 2023 ACC Expert Consensus Decision Pathway on Management of Heart Failure With Preserved Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2023;81(18):1835-1878. doi:10.1016/j.jacc.2023.03.393
Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022 May 3;79(17):e263-e421. doi: 10.1016/j.jacc.2021.12.012. Epub 2022 Apr 1. PMID: 35379503.
Bauersachs J, de Boer RA, Lindenfeld J, Bozkurt B. The year in cardiovascular medicine 2021: heart failure and cardiomyopathies. Eur Heart J. 2022 Feb 3;43(5):367-376. doi: 10.1093/eurheartj/ehab887. PMID: 34974611.
Gevaert AB, Kataria R, Zannad F, et al. Heart failure with preserved ejection fraction: recent concepts in diagnosis, mechanisms and management. Heart. 2022 Aug 11;108(17):1342-1350. doi: 10.1136/heartjnl-2021-319605. PMID: 35022210.
Voors A. Empagliflozin in Patients Hospitalized for Acute Heart Failure – EMPULSE presented at: American Heart Association Virtual Annual Scientific Sessions (AHA 2021), November 14, 2021.
McDonagh TA, Metra M, Adamo M, et al; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021 Sep 21;42(36):3599-3726. doi: 10.1093/eurheartj/ehab368. Erratum in: Eur Heart J. 2021 Oct 14; PMID: 34447992.
Van Spall HGC, Averbuch T, Damman K, Voors AA. Risk and risk reduction in trials of heart failure with reduced ejection fraction: absolute or relative? Eur J Heart Fail. 2021 Sep;23(9):1437-1444. doi: 10.1002/ejhf.2248. Epub 2021 Jun 16. PMID: 34041823.
Jering KS, Claggett B, Pfeffer MA, et al. Prospective ARNI vs. ACE inhibitor trial to Determine Superiority in reducing heart failure Events after Myocardial Infarction (PARADISE-MI): design and baseline characteristics. Eur J Heart Fail. 2021 Jun;23(6):1040-1048. doi: 10.1002/ejhf.2191. PMID: 33847047.
McDonald M, Virani S, Chan M, et al. CCS/CHFS Heart Failure Guidelines Update: Defining a New Pharmacologic Standard of Care for Heart Failure With Reduced Ejection Fraction. Can J Cardiol. 2021 Apr;37(4):531-546. doi: 10.1016/j.cjca.2021.01.017. PMID: 33827756.
Tromp J, Ponikowski P, Salsali A, et al. Sodium-glucose co-transporter 2 inhibition in patients hospitalized for acute decompensated heart failure: rationale for and design of the EMPULSE trial. Eur J Heart Fail. 2021 May;23(5):826-834. doi: 10.1002/ejhf.2137. Epub 2021 Mar 10. PMID: 33609072; PMCID: PMC8358952.
Bozkurt B, Coats AJ, Tsutsui H, et al. Universal Definition and Classification of Heart Failure: A Report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J Card Fail. 2021 Mar 1;S1071-9164(21)00050-6. doi: 10.1016/j.cardfail.2021.01.022. Online ahead of print. PMID: 33663906.
DefinitionTop
Definition and classification of heart failure (HF): see Heart Failure.
Epidemiology, Etiology, Pathophysiology Top
Heart failure with reduced ejection fraction (HFrEF) is better understood than heart failure with preserved ejection fraction (HFpEF) and represents approximately half of all HF cases. The most common etiology of HFrEF is coronary artery disease (CAD) and arterial hypertension. Other causes include viral infection, alcohol abuse, valvular heart disease, chemotherapy (eg, doxorubicin or trastuzumab), peripartum cardiomyopathy, “idiopathic” dilated cardiomyopathy, and genetic cardiomyopathies.
In HFrEF, maladaptive changes after myocardial injury lead to pathologic remodeling of the ventricle with dilatation and impaired contractility. This is mediated by:
1) Maladaptive left ventricle (LV) hypertrophy associated with the reexpression of fetal isoforms of contractile proteins and progressive loss of cardiomyocytes via apoptosis or necrosis.
2) Abnormal calcium homeostasis and depressed beta-receptor density in cardiomyocytes.
3) Myocardial fibrosis.
4) Progression of mitral insufficiency and pulmonary hypertension.
5) Neurohormonal activation involving the renin-angiotensin-aldosterone system (RAAS), sympathetic nervous system, and enhanced vasopressin secretion accompanied by impaired vasodilatory and renal responses to natriuretic peptides, which increases preload and afterload. These systemic neurohormonal responses are triggered by cardiac output lowering, have detrimental long-term effects on the heart and systemic vasculature, and create a vicious cycle of further myocardial injury and remodeling, which in turn exacerbates neurohormonal activation.
Additionally, the following systemic abnormalities both underlie HF symptoms and contribute to HF progression: depressed sensitivity of baroreceptors, enhanced reactivity of chemoreceptors and muscular ergoreceptors, vascular endothelial dysfunction and impaired vasodilatory reserve, renal dysfunction, skeletal muscle dysfunction, functional or absolute iron deficiency with or without anemia, chronic inflammatory activation, and neuroendocrine dysfunction favoring catabolic pathways.
HFpEF (left ventricular ejection fraction [LVEF] ≥50%) is highly prevalent, accounting for up to 50% of all patients with HF, and is associated with significant morbidity and mortality. HFpEF is a heterogenous disorder contributed to by comorbidities that include hypertension, diabetes mellitus (DM), obesity, CAD, chronic kidney disease, and specific causes (eg, cardiac amyloidosis). Pathophysiology involves heterogeneous factors, such as impaired LV relaxation due to excessive afterload (owing to elevated blood pressure [BP], aortic stenosis, increased arterial stiffness, and impaired peripheral vasodilatory reserve), decreased passive LV compliance (mediated by changes in the extracellular matrix associated with concentric LV hypertrophy), chronotropic incompetence, microvascular coronary dysfunction, and accompanying myocardial oxidative stress with consequent stimulation of proinflammatory and profibrotic pathways.
Causes of high-output HF include conditions associated with hyperdynamic circulation: severe anemia, thyrotoxicosis, large systemic arteriovenous fistulas, advanced cirrhosis, polycythemia or secondary erythrocytosis, Paget disease, beriberi, carcinoid syndrome, or pregnancy. HF usually develops in patients with hyperdynamic circulation superimposed on an underlying heart disease, although it can occur in absence of structural heart disease.
Cardiomyopathies (abnormalities in cardiac myocytes that result in impaired function) are a predisposing condition for clinical HF but may not always be associated with clinical HF. The World Health Organization (WHO) categorizes the etiology of HF based on the type of underlying cardiomyopathy, which is defined as pathologic myocardial processes and dysfunction that are a direct consequence of cardiovascular abnormalities other than valvular heart disease, systemic hypertension, congenital heart disease, and atherosclerotic coronary artery disease. For example, dilated cardiomyopathy may be due to toxins (eg, alcohol, cocaine, and chemotherapeutic agents such as anthracyclines, fluorouracil, and trastuzumab), myocarditis, Chagas disease, peripartum cardiomyopathy, and familial cardiomyopathies (eg, Becker or Duchenne muscular dystrophy), among others. Restrictive cardiomyopathies may be primary or secondary, with an example being cardiac amyloidosis with extracellular myocardial protein deposition, most commonly monoclonal immunoglobulin light chains (amyloid cardiomyopathy) or transthyretin amyloidosis. Patients present with usual HF symptoms but there may be a noticeable discordance between wall thickness on echocardiogram and QRS voltage on electrocardiography (ECG) and it may occur in the context of aortic stenosis, HFpEF, carpal tunnel syndrome, spinal stenosis or sensory polyneuropathy. Also see Cardiomyopathies.
Causes of HF exacerbations: Patients with chronic HF may develop exacerbations due to the following reasons:
1) Acute coronary syndrome.
2) Poorly controlled hypertension.
3) Tachyarrhythmia (most commonly AF) or bradyarrhythmia.
4) Pulmonary embolism.
5) Endocarditis, myocarditis.
6) Conditions associated with hyperdynamic circulation.
7) Infections (particularly pneumonia).
8) Renal dysfunction.
9) Dietary indiscretion (high sodium intake).
10) Nonadherence to prescribed medications.
11) Iatrogenic: IV fluids, use of medications with negative chronotropic or inotropic effects (eg, verapamil, diltiazem, or inappropriate doses of beta-blockers), cardiotoxic agents (eg, anthracyclines), drugs causing sodium and/or water retention (eg, glucocorticoids, estrogens, nonsteroidal anti-inflammatory drugs [NSAIDs]).
12) Abnormalities in thyroid function (eg, caused by amiodarone).
13) Alcohol abuse, cocaine use.
14) Untreated sleep apnea.
Clinical FeaturesTop
Symptoms of HF are a combination of pulmonary congestion, systemic congestion, or both with or without low output. The New York Heart Association (NYHA) classification of the severity of HF (functional status) is based on the assessment of fatigue, dyspnea, and palpitations, which are caused by physical activity (Table 3.8-1). A validated scale, such as that by the NYHA, is commonly used to document functional capacity.
1. Clinical manifestations of LV failure (pulmonary congestion):
1) Symptoms: Dyspnea (at rest or on exertion); orthopnea (occurs 1-2 minutes after lying down and resolves within a few minutes of sitting or standing up); paroxysmal nocturnal dyspnea, which unlike orthopnea occurs much later after lying down, wakes the patient up, and takes much more time (usually ≥30 minutes) to resolve; cough (an equivalent of exertional dyspnea or orthopnea), generally dry, occasionally producing pink sputum (usually in patients with pulmonary edema); fatigue.
2) Signs: Dilated LV or displaced apex with a third heart sound; crackles over the lung fields (typically audible in the basal regions, although they may extend up to the apical regions), which may be accompanied by wheezing and rhonchi (caused in part by edema of the bronchial mucosa).
2. Clinical manifestations of right ventricular failure (systemic congestion):
1) Symptoms: Peripheral dependent edema (most commonly affecting the feet and ankles, or the sacral area in bedridden patients), abdominal pain or discomfort caused by liver congestion, or nocturia. The symptoms may also include anorexia, nausea, and constipation caused by venous congestion of the gastric and intestinal mucosa and by reduced cardiac output, which in some cases may lead to malabsorption and subsequent malnutrition or even cachexia in patients with advanced HF.
2) Signs: Exudates, including pleural effusions (usually bilateral; when unilateral, they occur more frequently on the right) or ascites; right ventricular heave or a right ventricle that is palpable in the subxiphoid region; an enlarged and tender liver (tenderness is due to stretching of the hepatic capsule and occurs when congestion develops rapidly); a firm and atrophic liver may be observed in a long-standing (several years-long) HF. Other signs include mild jaundice, jugular vein distention with or without sustained elevation, and in some patients Kussmaul sign (increased jugular venous pressure during inspiration, similar to that observed in constrictive pericarditis).
3. Manifestations common to right and left ventricular failure (including manifestations of low cardiac output):
1) Symptoms: Impaired exercise tolerance, oliguria (in advanced HF).
2) Signs: Pale and cool skin of the extremities, diaphoresis, rarely acrocyanosis (features of sympathetic stimulation); tachycardia, third heart sound (often audible in patients with systolic LV dysfunction) or fourth heart sound (more suggestive of isolated diastolic HF), accentuated pulmonary component of the second heart sound; occasionally murmurs associated with valvular heart disease, which is a cause of HF or is secondary to ventricular dilatation; low pulse pressure, mild elevation of diastolic BP; Cheyne-Stokes respiration; symptoms of abnormal cerebral perfusion, especially in the elderly.
4. Symptoms and signs of HF with an increased cardiac output caused by hyperdynamic circulation: High pulse pressure (reduction in diastolic BP); increased precordial pulsation; pulsus altus et celer (bounding and rapid), capillary pulsation (Quincke pulse); tachycardia; abnormal features on auscultation (hyperdynamic heart sounds, sometimes a third or fourth heart sound, midsystolic ejection murmur audible along the left sternal border, sometimes midsystolic murmur over the mitral or tricuspid valve and constant venous hum, carotid bruit); warming and erythema of the skin (not present in patients with ischemia; it may be local, eg, in patients with Paget disease or arteriovenous fistulas). In patients with arteriovenous fistulas applying pressure on a fistula causes a decrease in heart rate.
5. Clinical features of HFpEF are similar to those of HFrEF and include exertional dyspnea and other symptoms of pulmonary congestion, while features of peripheral hypoperfusion are usually absent.
DiagnosisTop
A thorough history and physical examination are essential to screen for conditions that cause or precipitate HF.
Basic laboratory investigations are used to establish the cause of HF, detect associated complications, or ascertain prognosis. Chest radiography and echocardiography play a key role in diagnosing HF.
1. Laboratory investigations:
1) Hyponatremia caused by volume overload may be observed in patients with advanced HF or those who are treated with thiazide diuretics.
2) Hypokalemia or hyperkalemia and increased serum creatinine levels may be caused by diuresis, kidney injury, and/or adverse effects of drugs (see below).
3) Elevated serum aminotransferase, lactate dehydrogenase, and bilirubin levels are observed in patients with hepatic congestion.
4) Anemia (worsening or triggering HF) or high hematocrit values (eg, in chronic obstructive pulmonary disease and congenital heart disease with right-to-left shunt).
5) Serum levels of the natriuretic peptides can be measured to diagnose patients with a clinical suspicion of HF (especially in case of diagnostic uncertainty and unavailability of bedside echocardiography, which is increasingly being used in clinical practice). Due to a high negative but limited positive predictive value, natriuretic peptides are recommended to exclude HF but not to establish HF diagnosis. Exclusionary cutoff points for natriuretic peptides are higher in the acute than in the nonacute setting:
a) HF is unlikely in patients with no acute worsening of the symptoms (ie, in the nonacute setting), B-type natriuretic peptide (BNP) levels <35 pg/mL, N-terminal pro–B-type natriuretic peptide (NT-proBNP) levels <125 pg/mL, and normal ECG.
b) In patients with rapidly worsening symptoms (ie, in the acute setting), the cutoff points are as follows: BNP <100 pg/mL, NT-proBNP <300 pg/mL, midregional proatrial natriuretic peptide (MR-proANP) <120 pmol/L.
c) BNP/NT-proBNP can be used for prognostic stratification in patients with known HF. In ambulatory patients aged <75 years with low EF, BNP/NT-proBNP can be a useful adjunct to guide management.Evidence 1Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due to indirectness. Savarese G, Trimarco B, Dellegrottaglie S, et al. Natriuretic peptide-guided therapy in chronic heart failure: a meta-analysis of 2686 patients in 12 randomized trials. PLoS One. 2013;8(3):e58287. doi: 10.1371/journal.pone.0058287. Erratum in: PLoS One. 2014;9(4):e96706. PMID: 23472172; PMCID: PMC3589263. Troughton RW, Frampton CM, Brunner-La Rocca HP, et al. Effect of B-type natriuretic peptide-guided treatment of chronic heart failure on total mortality and hospitalization: an individual patient meta-analysis. Eur Heart J. 2014 Jun 14;35(23):1559-67. doi: 10.1093/eurheartj/ehu090. Review. PMID: 24603309; PMCID: PMC4057643.
It should be noted that (1) because natriuretic peptides tend to be lower in HFpEF than in HFrEF, the BNP/NT-proBNP threshold for diagnosis of HF in HFpEF is lower than in HFrEF; (2) BNP, but not NT-proBNP, is a substrate of neprilysin. Thus, BNP levels cannot be used to assess HF severity or treatment response in patients on angiotensin receptor–neprilysin inhibitors (ARNIs); NT-proBNP, however, can be used for these purposes in patients on ARNIs. Of note, NT-pro BNP levels are elevated in the presence of advanced age and renal failure, and slightly lower in individuals with a high body mass index (BMI).
2. ECG can reveal clues about the underlying etiology: ischemic heart disease, arrhythmia, conduction disturbances, or ventricular hypertrophy.
3. Chest radiography usually reveals an enlarged cardiac silhouette (except for the majority of patients with hyperdynamic circulation or HFpEF) and features of pulmonary congestion. It helps identify pulmonary edema while excluding other lung diseases that may be contributing.
4. Echocardiography is a key diagnostic study in HF and is usually performed in patients with suspected HF as it allows the assessment of:
1) LV systolic function through the analysis of global and segmental LV contractility and measurements of LVEF.
2) LV diastolic function through the analysis of tissue Doppler indices, left atrial volume, estimated pulmonary pressure, and presence of predisposing conditions.
3) Structural heart disease: Ventricular and/or atrial hypertrophy or dilatation, valvular heart disease, congenital heart disease, pericardial effusion.
In some cases (eg, a poor acoustic window in transthoracic echocardiography [TTE], significant native or prosthetic valve dysfunction, suspected thrombus in the left atrial appendage in patients with AF, diagnosis of bacterial endocarditis or congenital heart disease), transesophageal echocardiography (TEE) may be indicated.
Dobutamine stress echocardiography is useful in the diagnosis of coronary artery disease and can be considered in patients with intermediate pretest probability or for the assessment of reversible ischemia before proceeding to coronary angiography and revascularization.
5. Coronary angiography is indicated in patients with suspected ischemic heart disease, history of unexplained cardiac arrest, severe ventricular arrhythmias if myocardial ischemia is suspected as a cause, HF that is treatment resistant or of unknown etiology, or prior to elective cardiac surgery.
6. Exercise stress testing may be useful in patients with a discrepancy between the severity of symptoms and signs of the disease, in patients qualified for heart transplant, and to differentiate between cardiac and pulmonary causes of dyspnea.
7. Multislice CT (MSCT) and cardiac MRI may be used in determining the etiology of HF and in differential diagnosis when other methods (especially echocardiography and coronary angiography) are not sufficient to make a diagnosis. MSCT and MRI are particularly useful when differentiating between various forms of cardiomyopathy, cardiac tumors, pericardial disease, and congenital heart disease.
8. Endomyocardial biopsy has utility in selected patients with HF of unexplained etiology and with a suspected disease requiring specific treatment, including myocarditis (giant cell or eosinophilic), infiltrative or storage diseases (amyloidosis, sarcoidosis, hemochromatosis, or Fabry disease), and in diagnostics of transplant rejection.
HF is diagnosed in patients with typical signs and symptoms accompanied by objective features of systolic or significant diastolic LV dysfunction at rest, usually observed in echocardiography. Chest radiography criteria include interstitial or pulmonary edema. Another important indicator of the dysfunction is an increase in serum levels of natriuretic peptides (see Diagnostic Tests, above) and clinical improvement in response to standard HF pharmacotherapy. The Boston (medicalcriteria.com) or Framingham scoring criteria (mdcalc.com) offer a degree of objectivity to facilitate the diagnosis of HF.
Differential diagnosis includes other causes of dyspnea, edema, and jugular vein distension.
TreatmentTop
Treatment of HF includes:
1) Definitive treatment of the underlying condition (eg, coronary revascularization, valvular surgery).
2) Long-term treatment of HF (see below).
3) Treatment of HF exacerbations (see Acute Heart Failure).
1. In patients with symptoms of sodium and fluid retention, sodium restriction to 2 to 3 g/d is usually recommended (<2 g/d if the symptoms persist, especially if they are resistant to diuretic treatment). In patients with hyponatremia and severe symptoms (NYHA classes III-IV), add fluid restriction of 1.5 to 2 L/d.
2. Regular body weight monitoring:
1) Monitor the patient’s body weight (use the same scale for repeated measurements). An increase >2 kg in body weight over 3 days may indicate fluid retention caused by HF.
2) Reduce body weight in patients with obesity, especially those with a BMI >35 kg/m2.
3) Improve nutrition in patients with features of malnutrition (BMI <22 kg/m2, <90% of ideal body weight).
3. Reduction in alcohol consumption to ≤10 to 12 g/d in women and ≤20 to 25 g/d in men. Abstinence is necessary in patients with suspected alcohol cardiomyopathy.
4. Smoking cessation.
5. In patients with HF who have recently been hospitalized for an acute HF exacerbation, nurse home visits, nurse case management, and outpatient disease management/HF clinics should be considered, as they reduce all-cause mortality and readmissions.Evidence 2Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due to heterogeneity of interventions and results. Feltner C, Jones CD, Cené CW, et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta-analysis. Ann Intern Med. 2014 Jun 3;160(11):774-84. doi: 10.7326/M14-0083. Review. PubMed PMID: 24862840. Van Spall HGC, Rahman T, Mytton O, et al. Comparative effectiveness of transitional care services in patients discharged from the hospital with heart failure: a systematic review and network meta-analysis. Eur J Heart Fail. 19(11):1427-1443. doi:10.1002/ejhf.765. PMID: 28233442.
6. Avoidance of the following drugs (if possible):
1) NSAIDs, including cyclooxygenase-2 (COX-2) inhibitors (these increase fluid retention; reduce the effectiveness of diuretics, angiotensin-converting enzyme inhibitors [ACEIs], angiotensin receptor blockers [ARBs], and mineralocorticoid receptor antagonists [MRAs]; and increase the risk of adverse effects of these classes of drugs).
2) Glucocorticoids (these increase fluid retention and the risk of hypokalemia).
3) Class I antiarrhythmic drugs (particularly class Ia and Ic agents) and class III sotalol and ibutilide (see Table 3.4-1).
4) Tricyclic antidepressants (TCAs) (these have proarrhythmic effects and are associated with a risk of hypotension and HF exacerbation; selective serotonin reuptake inhibitors are relatively safe).
5) Dronedarone is contraindicated in patients with HF, as it increases cardiovascular mortality and risk of HF exacerbation.Evidence 3Strong recommendation (downsides clearly outweigh benefits; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of the intervention). Køber L, Torp-Pedersen C, McMurray JJ, et al; Dronedarone Study Group. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med. 2008 Jun 19;358(25):2678-87. doi: 10.1056/NEJMoa0800456. Erratum in: N Engl J Med. 2010 Sep 30;363(14):1384. PMID: 18565860.
6) Verapamil and diltiazem (except in patients with HFpEF; high doses of verapamil are routinely used in patients with hypertrophic cardiomyopathy); dihydropyridine calcium-channel blockers (in patients with coexisting hypertension or angina, long-acting agents [amlodipine and felodipine] may be used). Nondihydropyridine calcium-channel blockers are usually avoided in patients with HFrEF, given their negative inotropic effect.
7) Alpha1-blockers (these cause fluid retention and increase the risk of hypotension; in the case of voiding problems caused by symptomatic prostatic hyperplasia, switch to 5alpha-reductase inhibitors).
8) Moxonidine (increases the risk of death).
9) Metformin should be avoided in patients with contraindications (risk of lactic acidosis in patients with acute HF or severe respiratory, liver, or renal failure; it is commonly used and considered the first-line antidiabetic drug in patients with stable HF).
10) Thiazolidinediones (rosiglitazone, pioglitazone; these increase fluid retention and are absolutely contraindicated in NYHA class III or IV).Evidence 4Strong recommendation (downsides clearly outweigh benefits; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of the intervention). Lago RM, Singh PP, Nesto RW. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet. 2007 Sep 29;370(9593):1129-36. Review. PMID: 17905165. Home PD, Pocock SJ, Beck-Nielsen H, et al; RECORD Study Team. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009 Jun 20;373(9681):2125-35. doi: 10.1016/S0140-6736(09)60953-3. PMID: 19501900.
11) Anthracyclines (in the case of life-saving therapy consider using liposomal doxorubicin or pretreatment with dexrazoxane; closely monitor LV function).
7. Annual influenza and pneumococcal vaccination. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination according to local regulations.
8. Regular moderate physical activity: 30 minutes of aerobic exercise ≥3 times a week (in clinically stable patients with HF).
9. Caution is advised when traveling to altitudes >1500 meters above sea level or to hot and humid climates. In the case of long-distance journeys, air travel is preferred over land travel to avoid prolonged periods of immobilization.
10. Diagnosis and treatment of clinically significant depression (see Depressive Disorders).
11. In the case of concomitant central sleep apnea, consider continuous positive airway pressure (see Obstructive Sleep Apnea).
Pharmacotherapy of HFrEF: Figure 3.8-3.
General recommendations: Start with low doses of drugs and titrate them up to a target dose that has been proven effective in clinical trials or up to the maximum tolerated dose within 3 to 6 months from diagnosis.
Pharmacotherapy: First-Line Agents
In patients with HFrEF start treatment with guideline-directed medical therapy (GDMT) consisting of ARNI (or ACEI/ARB), low-dose beta-blockers (after HF is stabilized), MRAs, and a sodium-glucose cotransporter 2 (SGLT2) inhibitor.
1. Beta-blockers: Evidence supports the use of bisoprolol, carvedilol, and extended-release metoprolol. They should be used in patients with NYHA classes II to IV and LVEF ≤40% or in patients with asymptomatic systolic LV dysfunction, particularly after myocardial infarction (MI).Evidence 5Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of the intervention). Foody JM, Farrell MH, Krumholz HM. beta-Blocker therapy in heart failure: scientific review. JAMA. 2002 Feb 20;287(7):883-9. Review. PMID: 11851582. Treatment with a beta-blocker may be started with caution in patients with a recent history of HF decompensation as long as their condition has improved as a result of other treatments and the use of ongoing IV inotropic agents is not required. Agents and doses: Table 3.8-2.
General recommendations: During dose titration follow-up visits should be scheduled every 2 to 4 weeks, and the dose should be doubled at each visit as tolerated. Do not increase the dose in case of symptoms of HF exacerbation, symptomatic hypotension (eg, dizziness), or bradycardia <50 beats/min.
Beta-blockers should be reinitiated before discharge (if feasible) in hospitalized patients in whom they may have been stopped due to worsening HF.
Management of adverse effects:
1) Symptomatic hypotension should elicit a reassessment of diuretic dose with a view to minimize volume depletion, hypoperfusion, or both. Consider discontinuing antihypertensive agents that are not evidence-informed therapies for HFrEF. Stagger the dose of essential HF drugs such as ARNIs (or ACEIs, ARBs) and beta-blockers.
2) HF exacerbation: Increase the dose of diuretics (frequently a temporary increase is sufficient) and continue the beta-blocker treatment, if possible. In case of cardiogenic shock or hypoperfusion, address volume status, withhold the beta-blocker, and, if appropriate, use an IV inotropic agent such as milrinone or dobutamine (Table 3.8-3).
3) Excessive bradycardia: Perform ECG (or Holter ECG) to exclude heart block. Consider discontinuation of other atrioventricular node–blocking agents such as digoxin or amiodarone if the patient is treated with them. Dose reduction or discontinuation of the beta-blocker may be necessary.
4) Reactive airway disease: If present, consider using an agent with a more selective beta-1 blockade such as bisoprolol.
2. ARNIs: Initiate these as first-line therapy in both ambulatory and hospitalized patients with HFrEF without prior exposure to ACEI or ARB and provided there are no contraindications.
Use ARNIs preferentially over ACEIs or ARBs in patients with HFrEF (LVEF <40%) who are symptomatic (NYHA classes II-IV).Evidence 6Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due to lack of long-term follow up and single-trial data. McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014 Sep 11;371(11):993-1004. doi: 10.1056/NEJMoa1409077. PMID: 25176015. Patients on target-dose ACEIs and still symptomatic should be converted to the equivalent dose of ARNIs after a washout period of 36 hours (see below). Patients who do not tolerate ARNIs may be switched back to ACEIs after a 36-hour washout period. The role of ARNIs is rapidly evolving.
HF hospitalizations represent an opportunity to reassess and optimize therapy. Patients with HFrEF who are hospitalized for acute decompensated HF could be switched from ACEI/ARB to ARNI when stabilized and before discharge.Evidence 7Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due to indirectness, heterogeneity of effect, and imprecision. Velazquez EJ, Morrow DA, DeVore AD, et al; PIONEER-HF Investigators. Angiotensin–Neprilysin Inhibition in Acute Decompensated Heart Failure. N Engl J Med. 2019 Feb 7;380(6):539-548. doi: 10.1056/NEJMoa1812851. PMID: 30415601.
ARNIs are not preferentially recommended over ACEIs in the setting of acute MI. However, if other indications are present, ARNIs can be safely initiated in this setting.
The advantage of ARNIs over ARBs among patients with most advanced HF (NYHA class IV) is under investigation and remains unclear.
ARNIs are contraindicated in patients with hypotension (systolic BP ≤95 mm Hg), significant renal impairment (estimated glomerular filtration [eGFR] rate ≤30 mL/min/1.73 m2), hyperkalemia ([K+] ≥5.2 mmol/L), or severe hepatic disease (liver enzymes >2 × upper limit of normal). Serum levels of [K+] and creatinine should be closely monitored, but therapies can be continued and optimized until these thresholds of K+ and eGFR are met.
Ensure that patients are not concomitantly on ACEI (a 36-hour washout period from ACEIs is required to reduce the risk of angioedema).
Agents and dosage: Table 3.8-4. Doses can be uptitrated every 2 to 4 weeks.
3. ACEIs and ARBs: Initiate these in patients with acute MI with HF, LVEF ≤40% post MI (regardless of HF), those with HFrEF who are intolerant to ARNIs.Evidence 8Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of the intervention). Flather MD, Yusuf S, Køber L, et al. Long-term ACE-inhibitor therapy in patients with heart failure or left-ventricular dysfunction: a systematic overview of data from individual patients. ACE-Inhibitor Myocardial Infarction Collaborative Group. Lancet. 2000 May 6;355(9215):1575-81. PubMed PMID: 10821360. ARBs are typically initiated in patients who do not tolerate ACEIs because of persistent cough or angioedema,Evidence 9Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due to imprecision and heterogeneity. Heran BS, Musini VM, Bassett K, Taylor RS, Wright JM. Angiotensin receptor blockers for heart failure. Cochrane Database Syst Rev. 2012 Apr 18;(4):CD003040. doi: 10.1002/14651858.CD003040.pub2. Review. PMID: 22513909. Granger CB, McMurray JJ, Yusuf S, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003 Sep 6;362(9386):772-6. PMID: 13678870. although in such patients ARNI could be preferentially initiated. There are no significant differences in rates of hyperkalemia, renal dysfunction, or hypotension between ACEIs and ARBs. Do not use a combination of an ACEI and an ARB due to the risk of adverse events.
General recommendations and management of adverse effects for ACEIs and ARBs except for cough are listed below with other RAAS inhibitors. Agents and dosage: Table 3.8-4.
4. MRAs (eplerenone, spironolactone) should be used in patients withEvidence 10Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of the intervention). Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011 Jan 6;364(1):11-21. doi: 10.1056/NEJMoa1009492. PMID: 21073363. Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction [published correction appears in N Engl J Med. 2003 May 29;348(22):2271]. N Engl J Med. 2003;348(14):1309-1321. doi:10.1056/NEJMoa030207 Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999 Sep 2;341(10):709-17. PMID: 10471456.:
1) LVEF ≤40% and NYHA classes II to IV.
2) LVEF ≤40%, recent MI, and HF symptoms or DM.
They should be avoided in patients with eGFR <30 mL/min/1.73 m2.
Dosage: Table 3.8-5.
General recommendations:
1) Consider increasing the dose within weeks after starting treatment. Do not increase the dose in the case of significant deterioration of kidney function or hyperkalemia.
2) Monitor kidney function and serum electrolyte levels 1 and 4 weeks after starting treatment and after increasing the dose; then at 2, 3, 6, 9, and 12 months; and subsequently every 4 months.
3) Do not use MRAs in pregnant patients.
4) In patients with breast tenderness, breast enlargement, or both, switch from spironolactone to eplerenone.
Management of declining renal function or hyperkalemia in patients on RAAS inhibitors (ARNI, ACEI, ARB, MRA):
1) Serum urea nitrogen and creatinine levels may increase after the initiation of RAAS inhibitors; this is not clinically relevant unless rapid and severe. Among patients with diabetic kidney disease itself, RAAS inhibitors improve long-term renal outcomes despite associated decline in eGFR at initiation.
2) Monitor renal function and serum potassium within 2 to 4 weeks after initiating or changing the dose. If normokalemia is present and there is an increase <30% in creatinine, continue to titrate up the dose or continue with a maximum tolerated dose.
3) If there is an increase in creatinine or reduction in eGFR, evaluate for other etiologies of acute kidney disease (AKI) and correct for volume depletion. Reassess concomitant nephrotoxic medications and consider renal artery stenosis. Reduce the dose or stop ARNI/ACEI/ARB as a last option.
4) Avoid excessive diuretic treatment in all patients. Reassess volume status if there is deterioration of renal function.
5) If hyperkalemia is present, review concomitant medications that may be contributing to it, monitor potassium intake, and consider the addition of diuretics, sodium bicarbonate, and/or gastrointestinal cation exchangers. Reduce the dose or stop the RAAS inhibitors that triggered hyperkalemia as a last option.
6) Symptomatic hypotension (eg, dizziness) often resolves spontaneously even if the ARNI/ACEI/ARB dose is not changed. Check orthostatic vital signs to evaluate whether volume depletion is present. Consider reducing the dose of diuretics and concomitant antihypertensive agents that do not improve clinical outcomes. Stagger the doses of ARNI/ACEI/ARB with the beta-blocker.
7) Cough (in ~10% patients): Independent of the ACEI dose, cough usually appears within 1 week of ACEI treatment but may also occur after up to several months. It generally resolves within 3 to 5 days after discontinuation of ACEIs but may persist for up to a month or, in rare cases, even for several months. It usually recurs with the use of a different ACEI. If the cough is persistent and troublesome, switch to ARNI (or in the event of ARNI intolerance or hypotension, to ARB), if needed.
8) Angioedema (<1% patients): Switch to a different class of RAAS inhibitors (although on very rare occasions angioedema may also occur after ARBs).
5. SGLT2 inhibitors: SLGT2 inhibitors are agents classically used in the treatment of type 2 DM. Recently these drugs, mainly dapagliflozin and empagliflozin, have been shown to significantly reduce the risk of cardiovascular death and hospitalization due to HF in patients with HFrEF, with or without type 2 DM.Evidence 11Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). High Quality of Evidence (high confidence that we know true effects of the intervention). Cardoso R, Graffunder FP, Ternes CMP, Fernandes A, Rocha AV, Fernandes G, Bhatt DL. SGLT2 inhibitors decrease cardiovascular death and heart failure hospitalizations in patients with heart failure: A systematic review and meta-analysis. EClinicalMedicine. 2021 Jun 5;36:100933. doi: 10.1016/j.eclinm.2021.100933. PMID: 34308311; PMCID: PMC8257984. Li S, Vandvik PO, Lytvyn L, et al. SGLT-2 inhibitors or GLP-1 receptor agonists for adults with type 2 diabetes: a clinical practice guideline. BMJ. 2021 May 11;373:n1091. doi: 10.1136/bmj.n1091. PMID: 33975892. McMurray JJV, Solomon SD, Inzucchi SE, et al; DAPA-HF Trial Committees and Investigators. Dapagliflozin in Patients With Heart Failure and Reduced Ejection Fraction. The New England Journal of Medicine. N Engl J Med. 2019 Nov 21; 381(21):1995-2008. PMID: 31535829. Packer M, Anker SD, Butler J, et al; EMPEROR-Reduced Trial Investigators. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med. 2020 Oct 8; 383(15):1413-1424. PMID: 32865377.
General recommendations:
1) Initiate empagliflozin or dapagliflozin as first-line therapy along with other standard agents. The starting dose for both empagliflozin and dapagliflozin is 10 mg once daily.
2) Use SGLT2 inhibitors in patients with HFrEF, regardless whether type 2 DM is present, to improve quality of life and reduce the risk of both HF hospitalization and cardiovascular mortality. In addition, consider the use of these agents, even without clinically apparent HF, in:
a) Patients with type 2 DM or atherosclerotic cardiovascular disease to reduce the risk of death and HF hospitalization.
b) Patients aged >50 years with type 2 DM and risk factors for atherosclerotic cardiovascular disease to reduce the risk of HF hospitalization.
c) Patients with albuminuric renal disease to slow the progression of renal disease and reduce the risk of HF hospitalization.
3) SGLT2 inhibitors are currently contraindicated in patients with type 1 DM. The use of these agents in patients with HFrEF and eGFR <30 mL/min/1.73 m2 is not advised given the limited supporting evidence.
Dosage: Table 3.8-6.
Management of adverse effects of SGLT2 inhibitors:
1) Genital mycotic infections (GMIs): Patients at the highest risk of developing GMIs include women (10%-15% risk), those with a history of GMIs, and uncircumcised men. GMIs do not generally require discontinuation of SGLT2 inhibitors and can be managed with antifungal medications.
2) Hypoglycemia: A very rare event that typically occurs with concomitant use of insulin, a secretagogue, or both. Consider readjusting oral antidiabetic therapies to prevent occurrence.
3) Euglycemic diabetic ketoacidosis (DKA): The incidence of DKA associated with SGLT2 inhibitors is ~0.5 to 0.8 per 1000 patient-years. Blood glucose levels might be normal or moderately elevated (<14 mmol/L). Rarely patients may present with normal anion gap acidosis that can be detected by serum ketone measurement. Temporarily withhold SGLT2 inhibitors in patients who are unwell and develop volume depletion (eg, due to vomiting or diarrhea [“sick days”]) as well as in fasting states.
Patients taking SGLT2 inhibitors should be educated on the “sick day” management to reduce the risk of DKA. Assess volume status carefully when patients are on a combined regimen of diuretics, ARNIs, and SGLT2 inhibitors, as these agents promote diuresis.
Pharmacotherapy: Second-Line Agents
Consider adjuncts such as diuretics in patients with volume overload and ivabradine, vericiguat, digoxin, and a combination of hydralazine and isosorbide dinitrate in selected patients who may benefit from them.
1. Diuretics (loop diuretics with or without potassium-sparing diuretics) are used in patients with symptoms of volume overload and then continued at the lowest dose that prevents water retention. Agents and dosage: Table 3.8-5.
General recommendations:
1) Loop diuretics are generally the first-line agents in managing volume overload, particularly in symptomatic HF.
2) Adjust doses to the patient’s needs. Closely monitor the clinical status as well as serum levels of potassium, sodium, and creatinine.
3) Increase doses of diuretics until the signs and symptoms of volume overload improve (a recommended rate of body weight reduction is 0.5-1 kg/d). Adjust doses of diuretics (particularly when the desired dry body weight has been achieved) to avoid kidney injury and dehydration.
4) Encourage patients or caregivers to self-adjust the doses of diuretics based on the daily measurements of body weight and signs and symptoms of fluid retention.
5) In the case of resistance to diuretics, assess the patient’s adherence to the treatment regimen; make sure that the patient does not use NSAIDs, COX-2 inhibitors, glucocorticoids, cyclosporine (INN ciclosporin), or estrogens; assess the patient’s sodium and fluid intake; and then increase the dose of diuretics. If this is not effective, consider replacing furosemide with another loop diuretic (eg, bumetanide). At this point, administer the loop diuretic 2 times a day or when fasting, and finally consider a short-term IV infusion of a loop diuretic.
6) Ensure optimal afterload reduction, MRA use, and use of other guideline-recommended medical therapy.
Management of adverse effects:
1) In patients with hypokalemia/hypomagnesemia, start potassium or magnesium supplementation. Consider increasing the dose of ACEI/ARB or MRA for long-term prevention of hypokalemia/hypomagnesemia.
2) Hyperkalemia may occur in patients treated with ACEIs or ARBs in combination with potassium-sparing diuretics, including MRAs. Do not use potassium-sparing diuretics other than MRAs. Treatment may include use of oral ion exchangers (see Hyperkalemia). Patiromer and sodium zirconium cyclosilicate (SZC) are newer, much more costly, and possibly more biochemically efficient than the traditional sodium polystyrene sulfonate (SPS), but there is lack of direct comparison with older SPS and high-quality patient important benefit is not evident.
3) In patients with hyponatremia restrict fluid intake, discontinue the thiazide diuretic and substitute it (if possible) with a loop diuretic, and reduce the dose of or discontinue (if possible) the loop diuretic.
4) In patients with hyperuricemia and gout avoid NSAIDs.
5) In patients with possible hypovolemia determine the volume status and consider reducing doses of diuretics.
6) In patients with renal failure exclude hypovolemia and concomitant use of other nephrotoxic agents (eg, NSAIDs), then discontinue MRAs and thiazides (in patients treated with loop diuretics in combination with thiazides) and consider reduction of the dose of ACEI/ARB.
7) High-dose loop diuretics may be ototoxic.
2. The soluble guanylate cyclase (sGC) stimulator vericiguat may be considered as a second-line agent in addition to GDMT in selected patients with HFrEF (LVEF ≤45%), HF hospitalization within the previous 6 months, and worsening symptoms.Evidence 12Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due to indirectness and imprecision. Armstrong, PW, Burkert P, Anstrom KJ, et al. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med. 2020 May 14; 382(20), 1883-1893. doi: 10.1056/NEJMoa1915928. PMID: 32222134. Averbuch T, Van Spall HGC. Vericiguat reduced a composite of CV death or HF hospitalization in patients with HF and reduced LVEF. Ann Intern Med. 2020 Sep 15;173(6):JC30. doi: 10.7326/ACPJ202009150-030. PMID: 32926810.
Vericiguat is a novel sGC stimulator that enhances endogenous sGC sensitivity to nitric oxide and increases cyclic guanylate monophase production resulting in downstream beneficial effects on the cardiovascular system. The drug received approval from the US Food and Drug Administration (FDA) in 2021 and was approved in Canada in 2023.
General recommendations: Initiate a dose of 2.5 mg daily and titrate up to the target dose of 10 mg daily if tolerated. Vericiguat is contraindicated in patients with concomitant use of other sGC stimulators and in pregnant patients.
Dosage: Table 3.8-7.
3. Consider ivabradine,Evidence 13Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). Low Quality of Evidence (low confidence that we know true effects of the intervention). Quality of Evidence lowered due to indirectness and heterogeneity of results. Swedberg K, Komajda M, Böhm M, et al; SHIFT Investigators. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010 Sep 11;376(9744):875-85. doi: 10.1016/S0140-6736(10)61198-1. Erratum in: Lancet. 2010 Dec 11;376(9757):1988. Lajnscak, M [corrected to Lainscak, M]; Rabanedo, I Roldan [corrected to Rabadán, I Roldan]; Leva, M [corrected to Ieva, M]. PMID: 20801500. Fox K, Ford I, Steg PG, Tendera M, Ferrari R; BEAUTIFUL Investigators. Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a randomised, double-blind, placebo-controlled trial. Lancet. 2008 Sep 6;372(9641):807-16. doi: 10.1016/S0140-6736(08)61170-8. PMID: 18757088. which reduces the heart rate by having selective effects on the sinus node, in patients with:
1) LVEF ≤35%, preserved sinus rhythm ≥70 beats/min, and NYHA classes II to IV despite the use of ACEI (or ARB), MRA, and a beta-blocker at optimal doses.
2) Contraindications to or intolerance of beta-blockers.
General recommendations: Start with 5 mg bid and after 2 weeks increase the dose to 7.5 mg bid, provided the patient continues to be in sinus rhythm with a heart rate of >60 beats/min (treatment with ivabradine cannot be the reason for reducing the dose of a beta-blocker without a strong justification, as the benefit of ivabradine is in reducing readmissions [no effect on mortality]).
Management of adverse effects:
1) Asymptomatic sinus bradycardia <50 beats/min or symptomatic bradycardia: Reduce the dose to 2.5 mg bid. If the symptoms persist, discontinue ivabradine.
2) Bradycardia associated with hemodynamic instability: Discontinue ivabradine. Consider administration of a beta-agonist (eg, isoproterenol [INN isoprenaline]) or temporary pacing, if necessary.
3) Vision disturbances (transient sensation of bright light in a portion of the visual field, which may interfere with the ability to drive) usually occur in the first 2 months of treatment and generally resolve spontaneously. However, discontinuation of treatment must be considered in case of a sudden vision impairment.
Occurrence of AF or atrial flutter during treatment with ivabradine increases the risk of rapid ventricular rate if the patient does not receive concomitant beta-blockers or low-dose beta-blockers. Ivabradine should be discontinued ≥24 hours before an elective cardioversion.
4. Digoxin reduces the frequency of hospitalization with no survival benefit. It may be considered in patients with HF and LVEF ≤40% in sinus rhythm who remain symptomatic (NYHA classes II-IV) despite receiving treatment with ARNI or ACEI (or ARB in patients intolerant to either ARNIs or ACEIs), a beta-blocker, and MRA at optimal doses.Evidence 14Weak recommendation (benefits likely outweigh downsides, but the balance is close or uncertain; an alternative course of action may be better for some patients). Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due to indirectness (main trial was conducted in an era when beta-blockers and MRAs were not the mainstay of HF treatment). Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997 Feb 20;336(8):525-33. PMID: 9036306.
Once the clinical status is stabilized, digoxin is preferably used in combination with a beta-blocker. Digoxin may be used in stable patients with HFrEF and AF in whom beta-blockers are not tolerated, ineffective, or contraindicated. If the effects of the combined treatment with digoxin and a beta-blocker are insufficient for rate control in AF, you may switch from digoxin to amiodarone. Avoid the combination of digoxin, a beta-blocker, and amiodarone.
Digoxin has been associated with a higher absolute risk of all-cause death in women than in men, including women treated with digoxin having a higher rate of death than those assigned to placebo. There are no guidelines to address these sex-specific differences and research is warranted to further ascertain the safety of digoxin use in female patients with HF.
Contraindications: Hypertrophic cardiomyopathy with LV outflow tract obstruction, preexcitation syndromes, hypokalemia, hypercalcemia, life-threatening ventricular arrhythmia, cardiac amyloidosis (digoxin binds to amyloid), multifocal atrial tachycardia, patients prior to planned electric cardioversion, bradycardia, second-degree and third-degree atrioventricular blocks, sick sinus syndrome (unless the patient has an implanted pacemaker).
General recommendations:
1) Boluses of 0.25 to 0.5 mg IV (loading dose up to 1 mg over 8-24 h) may be used to slow down rapid ventricular rate in digoxin-naive patients with HF and AF in an acute setting followed by 0.125 to 0.25 mg/d. Loading doses are not recommended in hemodynamically stable patients with AF and in patients with preserved sinus rhythm. With routine maintenance doses (0.125-0.25 mg/d orally), steady state serum drug levels are achieved within ~7 days.
2) In elderly patients and in patients with impaired renal function, hypothyroidism, or low body weight, the maintenance doses are 0.0625 or 0.125 mg/d orally. Exercise caution with the loading dose in this population.
3) It has not been proven that regular serum digoxin level measurements are associated with improved outcomes (therapeutic range is 0.6-1.2 ng/mL).
4) Serum digoxin levels may be increased by amiodarone, diltiazem, verapamil, certain antibiotics (macrolides, tetracyclines), proton pump inhibitors, H2-blockers, and quinidine.
Management of adverse effects: see Digoxin and Other Cardiac Glycosides.
5. A fixed-dose combination of hydralazine (or dihydralazine) and isosorbide dinitrate: In patients with LVEF ≤35% (or LVEF ≤45% and LV dilation) this may be considered an alternative to ACEI/ARNI/ARB if none of these agents are tolerated due to renal dysfunction or hyperkalemia (in such cases use it in combination with a beta-blocker and MRA, if possible). Adding the combination to treatment may also be considered in patients who have persistent symptoms of HF when receiving treatment with standard GDMT and is recommended in Black patients with who remain symptomatic despite treatment with ARNI (ACEI/ARB), a beta-blocker, and MRA.
Consider a trial of this combination in patients with a significant change in creatinine from baseline or those with serum creatinine >220 mmol/L who have worsening renal function with the use of ARNI/ACEI/ARB therapy despite dose adjustment and removal of nephrotoxic medications. In addition, such a trial may also be considered in patients with persistent hyperkalemia ([K+] >5.5 mmol/L) despite dose reduction, removal of other agents that increase serum potassium levels, and dietary intervention.
Adverse effects: Symptomatic hypotension, tachycardia, arthralgia, myalgia, drug-induced lupus-like syndrome.
6. Antithrombotic treatment should be used in patients with additional indications, that is, paroxysmal or persistent AF or atrial flutter, intracardiac thrombus, or a history of peripheral embolism.
There are less convincing data to date for treatments that reduce morbidity or mortality in patients with HFpEF. The current standard of practice includes:
1) Optimal treatment of the underlying condition: This may involve rate control in the setting of AF, treatment of sleep apnea with continuous positive airway pressure, strict control of hypertension (preferably using agents that reduce LV remodeling [ACEI and ARB]), revascularization in CAD.
2) Restriction of sodium and fluid intake in patients with hyponatremia: Recent randomized controlled trials demonstrate that for patients with chronic symptomatic HF (NYHA II-III), strict fluid restriction does not enhance their health status.
3) SGLT2 inhibitors should be initiated in all HFpEF patients without contraindications, ideally once they are stable during initial hospitalization. SGLT2 inhibitors have been shown to decrease the risk of hospitalization in HF with mildly reduced EF and HFpEF (NYHA class II-IV symptoms, LVEF >40%) in comparison with placebo. Notably, these benefits were demonstrated regardless of DM history.Evidence 15Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). High Quality of Evidence (high confidence that we know true effects of the intervention). Solomon SD, McMurray JJV, Claggett B, et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N Engl J Med. 2022;387(12):1089-1098. doi:10.1056/NEJMoa2206286 Anker SD, Butler J, Filippatos G, et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N Engl J Med. 2021;385(16):1451-1461. doi:10.1056/NEJMoa2107038
4) Beta-blockers have not been adequately tested in HFpEF, but they are used to improve LV filling by extending diastole (target resting heart rates 60-70 beats/min, or 55-60 beats/min in patients with angina), particularly in patients after MI or with angina, either with sinus rhythm or with AF. Digoxin may be used in patients with AF and rapid ventricular rhythm or in patients with AF not responding to monotherapy with a beta-blocker, verapamil, or diltiazem (digoxin reduces mainly the resting heart rate, while beta-blockers reduce heart rates on exertion). Assess the feasibility of restoring sinus rhythm.
5) RAAS inhibitors: ACEI, ARB, MRA, and ARNI may be used to reduce the risk of HF hospitalization and appear to be effective until the threshold of ~55%. Sacubitril/valsartan in comparison with valsartan has been associated with a more favorable treatment effect in female patients with HFpEF than in male patients with HFpEF. Females with HFpEF are at a decreased risk of HF hospitalizations and cardiovascular death. Further post hoc analyses revealed that ACEI, MRA, or ARNI seem to have the most benefits in patients with HFrEF and HFpEF, with sex-specific treatment interactions such that the LVEF threshold of effectiveness is greater in females than in males.
6) Diuretics are used in patients with symptoms of fluid retention. Use these with caution to avoid excessive reduction in cardiac output and subsequent hypotension (measure supine and standing BP) as well as impairment of kidney function.
7) MRA: In patients with symptomatic HF with preserved or mildly reduced LVEF, finerenone resulted in a lower composite rate of worsening HF events (mostly) and cardiovascular death compared with placebo,Evidence 16Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due to indirectness. Solomon SD, McMurray JJV, Vaduganathan M, et al. Finerenone in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N Engl J Med. 2024;391(16):1475-1485. doi:10.1056/NEJMoa2407107 although no direct comparison with older MRA is available.
8) Glucagon-like peptide 1 (GLP-1) agonists: In patients with HFpEF and obesity, treatment with semaglutide led to larger reductions in symptoms and physical limitations, greater improvements in exercise function, and greater weight loss than placebo.
Consider referral to a specialized center.
1. Cardiac resynchronization therapy (CRT) involves placement of 2 electrodes to stimulate the right and left ventricles, and an additional electrode in the right atrium to synchronize the stimulation of the ventricles with the intrinsic atrial rhythm. CRT improves exercise tolerance, reduces the incidence of hospitalization caused by symptom exacerbation, and in patients with preserved sinus rhythm also reduces the risk of death.
Consider referral to a specialized center in patients who are clinically stable, have life expectancy >1 year with reasonable functional status, and (1) are symptomatic with EF ≤35%, and QRS ≥130 milliseconds in the case of left bundle branch block (LBBB); or (2) have QRS ≥150 milliseconds in the case of non-LBBB QRS morphology.
Patients with decreased EF may be candidates for CRT-D (CRT defibrillator; cardiac resynchronization + cardioverter-defibrillator). Therefore, when qualifying patients for CRT, we typically assess LVEF and the NYHA class after ≥3 months of optimal pharmacotherapy; in patients with ischemic heart disease, after >40 days post MI; and after >3 months post percutaneous coronary intervention (PCI).
2. Implantable cardioverter-defibrillator (ICD): These devices have been shown to reduce sudden cardiac death (SCD) and are typically considered in patients receiving optimal pharmacotherapy for ≥3 months, after revascularization (where indicated), and with >1-year life expectancy with good functional status. Consider referral to a specialized cardiac center for assessment in the following cases:
1) Primary prevention:
a) Post-MI systolic LV dysfunction (LVEF ≤35% measured >40 days after MI and >3 months after coronary revascularization, whenever performed) in patients with NYHA classes II to III.
b) NYHA classes II to III systolic LV dysfunction (LVEF ≤35%) of a nonischemic etiology (primary prevention of SCD).
2) Secondary prevention: History of ventricular fibrillation or ventricular tachycardia causing a loss of consciousness or hemodynamic instability regardless of LVEF, as long as these were not due to a transient or reversible condition, for instance, in the first 48 hours of MI.
In patients who meet the above criteria and have concomitant indications for CRT, implantation of a device with both CRT and ICD functions (CRT-D) is preferred.
3. Coronary revascularization is usually done in patients with HF caused by ischemic heart disease, particularly with symptoms of angina. In the absence of angina, establish indications for revascularization on the basis of documented ischemia of a viable myocardium supplied by an artery suitable for revascularization.
4. In patients refractory to medical, invasive, and device treatments, consider referral to a specialized center for heart transplant assessment. Left ventricular assist devices (LVADs) may be used when waiting for the transplant in patients with HFrEF.
RehabilitationTop
Participation in interval or continuous training programs is recommended in HF regardless of LVEF values as long as the patient is clinically stable and physical activity does not lead to exhaustion or trigger new HF symptoms.Evidence 17Strong recommendation (benefits clearly outweigh downsides; right action for all or almost all patients). Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due to low precision of estimates for all-cause mortality and unclear risk of bias. Taylor RS, Sagar VA, Davies EJ, et al. Exercise-based rehabilitation for heart failure. Cochrane Database Syst Rev. 2014 Apr 27;(4):CD003331. doi: 10.1002/14651858.CD003331.pub4. Review. PMID: 24771460. O'Connor CM, Whellan DJ, Lee KL, et al; HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009 Apr 8;301(14):1439-50. doi: 10.1001/jama.2009.454. PMID: 19351941; PMCID: PMC2916661. Isometric exercise is not recommended.
PrognosisTop
SCD accounts for up to 50% of all deaths among patients with HF. Improved prognosis in HF has been documented in:
1) Patients treated with ACEI/ARB/ARNI, beta-blockers, MRA, and a combination of hydralazine and isosorbide dinitrate (the last one in Black patients on contemporary treatments with other drugs). Diuretics in monotherapy have no effect on prognosis.
2) Certain subgroups of patients treated with CRT or ICD as well as patients after coronary revascularization.
Chronic HF is marked by periods of stability interspersed with exacerbations requiring hospitalization. Epidemiologic data from Medicare beneficiaries hospitalized for HF suggested a 30-day readmission risk of 25% and a 1-year mortality risk of 37%. Advanced HF is characterized by frequent exacerbations, intolerance of preventive therapies, metabolic perturbations, and overall deterioration that culminates in death.Evidence 18Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due to indirectness to today’s evolving treatment options. Curtis LH, Greiner MA, Hammill BG, et al. Early and long-term outcomes of heart failure in elderly persons, 2001-2005. Arch Intern Med. 2008 Dec 8;168(22):2481-8. doi: 10.1001/archinte.168.22.2481. PMID: 19064833; PMCID: PMC2629051. Meta-analysis Global Group in Chronic Heart Failure (MAGGIC). The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Eur Heart J. 2012 Jul;33(14):1750-7. doi: 10.1093/eurheartj/ehr254. Review. PMID: 21821849. In the current era of ICD use, SCD has been replaced by pump failure as a common mechanism of death.
Overall, patients with HFpEF may have a better prognosis than those with HFrEF. A meta-analysis of published trials in patients with HF found the 3-year all-cause mortality rate to be 32% in HFrEF (adjusted for prognostic risk factors) versus 24% in HFpEF.
Tables and FiguresTop
|
Class |
Functional capacity |
|
I |
No limitation of physical activity. Ordinary physical activity does not cause undue fatigue, dyspnea, or palpitation. |
|
II |
Slight limitation of physical activity. The patient is comfortable at rest, but ordinary physical activity results in fatigue, palpitation, or dyspnea. |
|
III |
Marked limitation of physical activity. The patient is comfortable at rest, but less than ordinary activity causes fatigue, palpitation, or dyspnea. |
|
IV |
Any physical activity causes discomfort. Symptoms of heart failure may be present even at rest. Discomfort increases with any physical activity. |
|
Based on The Criteria Committee of the New York Heart Association. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. 9th ed. Little, Brown & Co; 1994:253-256. |
|
|
Beta-blocker |
Starting dose (mg)a |
Subsequent doses up to target dose (mg) |
|
Bisoprolol |
1.25 |
2.5 to 3.75 to 5 to 7.5 to 10 |
|
Carvedilol |
3.125 |
6.25 to 12.5 to 25 to 50 |
|
Metoprolol succinate controlled-release |
12.5 or 25 |
25 to 50 to 100 to 200 |
|
a Carvedilol is administered 2 times a day, while other listed beta-blockers are administered once daily. The table lists single doses of agents. |
||
|
Agents |
Dosage |
Comments |
|
Dopamine |
1) <3 microg/kg/min 2) 3-5 microg/kg/min 3) >5 microg/kg/min (maximum, 30 microg/kg/min) |
– Low doses (1) mainly cause vasodilation of visceral, renal, and coronary vascular beds; intermediate doses (2) increase myocardial contractility and cardiac output via adrenergic stimulation; high doses (3) increase peripheral vascular resistance through alpha-adrenergic stimulation (this may cause clinical deterioration in patients with AHF due to an increase in LV and right ventricular afterloads) – May be used in patients with AHF and low BP – Low-dose dopamine is often used in combination with higher doses of dobutamine – Survival on dopamine may be worse compared with norepinephrine in case of cardiogenic shock (hypotension and hypoperfusion)Evidence 17Moderate Quality of Evidence (moderate confidence that we know true effects of the intervention). Quality of Evidence lowered due to subgroup analysis of a randomized controlled trial with unclear classification of the type of shock. De Backer D, Biston P, Devriendt J, et al; SOAP II Investigators. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010 Mar 4;362(9):779-89. doi: 10.1056/NEJMoa0907118. PubMed PMID: 20200382. |
|
Dobutamine |
2-20 microg/kg/min |
– Used to increase cardiac output – Stimulates beta1-receptors, increases myocardial contractility, increases heart rate; lower doses cause vasodilation while higher doses cause vasoconstriction – Infusion over >24-48 h is associated with development of tolerance and partial loss of hemodynamic effects – Discontinuation may be difficult because of recurrent low BP, congestion, or kidney failure, which is why infusion rates should be reduced gradually (by 2 microg/kg/min per day) and used together with optimized vasodilator therapy (eg, oral ACEI) – May cause ventricular or supraventricular arrhythmia and chest pain in patients with IHD |
|
Milrinone |
Bolus 25-75 microg/kg over 10-20 min followed by infusion of 0.375-0.75 microg/kg/min |
– Phosphodiesterase inhibitor (inhibits cAMP degradation); has positive inotropic effects and facilitates myocardial relaxation and vasodilation – Indicated in patients with peripheral hypoperfusion and normal BP with or without pulmonary congestion in whom diuretic and vasodilator treatment was ineffective – May be used instead of dopamine in patients treated with beta-blockers in the case of inadequate response to dobutamine – May have proarrhythmic effects and should be used with caution in patients with IHD |
|
Norepinephrine |
0.2-2 microg/kg/min |
– Use (with caution!) only in patients with cardiogenic shock and BP <90 mm Hg when organ perfusion is inadequate in spite of other interventions – May be indicated in shock patients with AHF and sepsis – May be combined with each of the above inotropic agents (use with caution in combination with dopamine) |
|
Epinephrine |
1 mg every 3-5 min (only during CPR); 0.05-0.5 microg/kg/min |
Use only during CPR in patients with cardiac arrest or in the case of dobutamine resistance and persistent hypotension |
|
Digoxin |
Loading dose 0.5-1 mg, followed by 0.125-0.375 mg/d (monitor serum drug levels) |
Effective in patients with AHF caused by tachyarrhythmia (eg, AF); not recommended in patients with AHF after acute MI because of its proarrhythmic effects |
|
ACEI, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; AHF, acute heart failure; BP, blood pressure; cAMP, cyclic adenosine monophosphate; CPR, cardiopulmonary resuscitation; IHD, ischemic heart disease; IV, intravenous; LV, left ventricular; MI, myocardial infarction. |
||
|
Agent |
Dose |
|
|
Starting |
Target |
|
|
ACEI |
||
|
Enalapril |
2.5 mg bid |
10-20 mg bid |
|
Captopril |
6.25 mg tid |
50 mg tid |
|
Lisinopril |
2.5-5 mg once daily |
20-35 mg once daily |
|
Ramipril |
2.5 mg once daily |
5 mg bid |
|
Trandolapril |
0.5 mg once daily |
4 mg once daily |
|
ARB |
||
|
Candesartan |
4 mg or 8 mg once daily |
32 mg once daily |
|
Valsartan |
40 mg bid |
160 mg bid |
|
Losartana |
50 mg once daily |
150 mg once daily |
|
ARNI |
||
|
Sacubitril/valsartanb |
24/26 mg bid |
97/103 mg bid |
|
Contraindications: table 3.9-3. a Losartan is mentioned in the 2012 European Society of Cardiology guidelines but it is stressed in the text that the benefits of using the agent may be lower. b Valsartan component in the sacubitril/valsartan combination pill Entresto is more potent than valsartan monotherapy pills available on the market. Therefore, caution must be exercised not to equate valsartan dosage found in current monotherapy with the dose found in Entresto, as it will lead to prescribing a higher than the intended dose. |
||
|
ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; ARNI, angiotensin receptor neprilysin inhibitor; bid, 2 times a day; tid, 3 times a day. |
||
|
Agent |
Starting dose (mg/d) |
Usual dose (mg/d) |
|
Loop diuretics | ||
|
Furosemidea |
20-40 |
40-240 |
|
Torasemide |
5-10 |
10-20 |
|
Thiazide and thiazide-like diuretics | ||
|
Chlorthalidone |
12.5-25 |
25-100 |
|
Hydrochlorothiazide |
25 |
12.5-100 |
|
Indapamide |
2.5 (1.5 mg when using controlled-release formulations) |
2.5-5 |
|
Potassium-sparing diureticsb | ||
|
Amiloride (available only in a fixed combination with hydrochlorothiazide) |
2.5 (5) |
5-10 (10-20) |
|
Eplerenone |
25 (50) |
50 (100) |
|
Spironolactone |
12.5-25 (50) |
50 (100-200) |
|
a The diuretic effect starts within 30-60 min, peaks in 1-2 h, and ends after 6-8 h. b Doses used in patients who are not treated with an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker are given in parenthesis. | ||
|
SGLT2 inhibitor |
Starting dose (mg) |
Target dose (mg) |
|
Empagliflozin |
10 mg once daily |
10 mg once daily |
|
Dapagliflozin |
10 mg once daily |
10 mg once daily |
|
SGLT2, sodium-glucose cotransporter 2. |
||
|
Agent |
Starting dose (mg) |
Target dose (mg) |
|
Vericiguat |
2.5 mg once daily |
10 mg once daily |
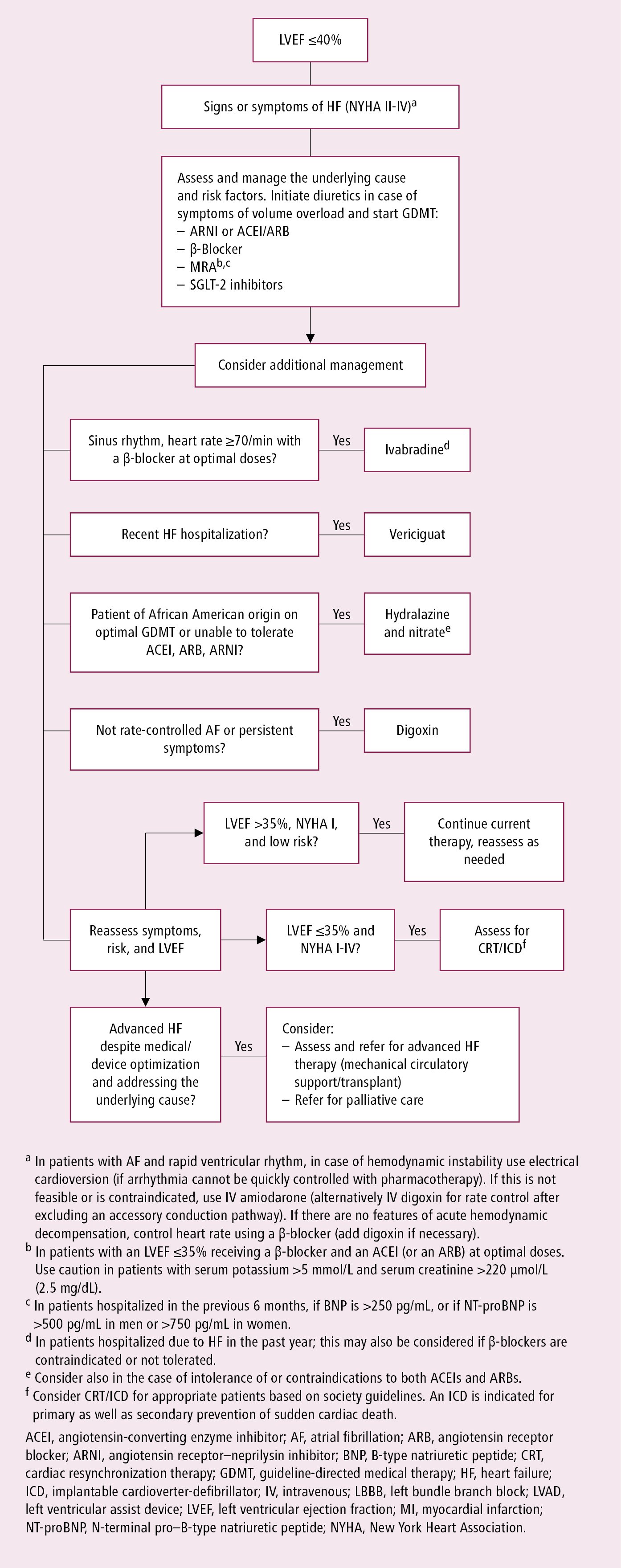
Figure 3.8-3. Suggested management of chronic heart failure. Based on Can J Cardiol. 2021 Apr 1;37(4):531-546.